Table of Contents
What is the difference between steric and torsional strain?
Let’s talk about steric strain and torsional strain, two important concepts in organic chemistry. They describe the instability of a molecule due to different kinds of repulsions between atoms.
The main difference is in how these strains can be minimized. Steric strain happens when atoms or groups of atoms are too close together. Think of it like trying to cram too many people into a small room—it’s uncomfortable! In contrast, torsional strain arises when atoms are twisted into an unfavorable conformation, like trying to force a person to sit in a chair that’s too small.
Now, here’s the key: while you can reduce torsional strain by rotating the molecule around a bond, steric strain is often unavoidable, even with rotation.
Imagine a chair with arms. Steric strain would be like having a person with large shoulders trying to fit into the chair. Even if you rotate the person, their shoulders will still bump against the chair’s arms.
On the other hand, torsional strain is like having a person trying to sit in the chair with their legs bent at an awkward angle. If they rotate their body, they can find a more comfortable position where their legs aren’t twisted.
Here’s a simpler analogy: Imagine holding a balloon. If you try to squeeze the balloon too tightly, that’s steric strain. The balloon will push back against your hand. However, if you twist the balloon, that’s torsional strain. You can reduce the twisting by rotating the balloon.
In essence, steric strain is about space—how close atoms are to each other—while torsional strain is about conformation—how the atoms are arranged in space.
So, while both types of strain contribute to a molecule’s instability, it’s important to remember that you can often minimize torsional strain, but steric strain is more persistent.
What is torsional strain?
Think of torsional strain as the resistance a molecule has to twisting. In cyclic molecules, meaning molecules shaped like rings, this kind of strain is also called Pitzer strain. You see, when atoms that are separated by three bonds are forced to be close together in an eclipsed conformation, they experience strain. This is because the electrons around those atoms are trying to stay as far away from each other as possible. They’d rather be in a staggered conformation, where they have more space to breathe.
So, the bottom line is: torsional strain is a form of energy that molecules have to deal with when they are forced to twist. It’s like a little bit of stress that makes the molecule less happy. If you want to imagine what eclipsed and staggered conformations look like, think about a propeller. Imagine the propeller blades are the bonds and the center of the propeller is the carbon atom. In the eclipsed conformation, the blades would be lined up with each other, creating a lot of “friction.” In the staggered conformation, the blades would be offset from each other, allowing for more space.
It’s important to remember that this strain is just one type of energy that molecules have to deal with. There are other kinds of strain, such as steric strain, which happens when atoms are too close together. But torsional strain is specifically about the resistance to twisting and how this can impact the stability of a molecule.
What is a steric strain?
Think of it like trying to squeeze too many people onto a small bus. Everyone gets uncomfortable, and it’s hard to move around! The same thing happens in molecules: when atoms get too close, they start pushing back on each other, making the molecule less stable.
Let’s break it down:
Non-bonded Interactions: These occur when atoms aren’t directly connected by a chemical bond. They’re like the “bumps” in the bus analogy.
Close Proximity: When atoms are very close to each other, their electron clouds overlap, and this overlap creates repulsion. It’s like when you put two magnets too close together—they push away from each other.
Repulsion: This repulsion between electron clouds is the root cause of steric strain. It creates a force that tries to push the atoms apart, making the molecule less stable.
Steric strain plays a crucial role in determining the shape and reactivity of molecules. Understanding it can help us predict how molecules will behave in different situations, which is essential for many areas of chemistry, like designing new drugs or understanding how enzymes work.
What is the difference between steric strain and gauche?
Let’s break this down further. Imagine two bulky groups like methyl groups (CH3) attached to a carbon chain. When these groups are positioned directly opposite each other, they are in the anti conformation. This is the most stable arrangement because the groups are as far apart as possible, minimizing their repulsive interactions.
Now, imagine rotating one of these groups by 60 degrees. This puts the groups in the gauche conformation. In this conformation, the groups are closer together, leading to a greater repulsive force between them. This increased repulsion makes the gauche conformation less stable than the anti conformation, resulting in a higher energy level.
The difference in energy between these conformations is due to steric strain. This strain arises from the non-bonded interactions between electron clouds of atoms or groups that are forced too close together. The closer the groups are, the greater the repulsive force and the higher the energy level.
Therefore, the anti conformation is favored due to its lower steric strain and lower energy level. Understanding these concepts is crucial for comprehending the behavior and reactivity of molecules, especially those containing bulky groups.
Does steric strain increase stability?
Increased steric strain actually decreases the stability of a bond. Imagine two big groups attached to a central atom. They’re constantly bumping into each other, making the molecule less stable. Think of it like trying to balance a book on a narrow edge – it’s much easier to tip over than a book balanced on a wide base.
So how does steric strain work in the real world? Take the example of conformations of cyclohexane. Cyclohexane is a six-membered ring, and it can adopt different shapes, or conformations. The most stable conformation is the chair conformation, where all the hydrogen atoms are spread out, minimizing steric strain. In contrast, the boat conformation has a much higher steric strain due to interactions between hydrogen atoms on the same side of the ring. This makes the boat conformation less stable than the chair conformation.
Steric strain is a crucial factor to consider when understanding the reactivity and properties of molecules. It can influence the rate of chemical reactions, affect the shape of molecules, and even determine the stability of different conformations. Understanding this concept is key to unlocking the mysteries of molecular behavior.
Is Van der Waals strain the same as steric strain?
Think of it like this: Imagine you have a bunch of bouncy balls packed tightly into a box. Each ball represents an atom, and the box represents the molecule. If you try to squeeze the box even tighter, the balls will start to push against each other, creating a kind of strain. This is analogous to steric strain in molecules.
The van der Waals radius is a measure of how close two non-bonded atoms can get before they start to repel each other. When atoms or groups are forced closer than their van der Waals radii allow, their electron clouds start to overlap, leading to increased repulsive forces and a rise in the potential energy of the molecule. This increase in potential energy is what we call steric strain.
Steric strain can have a significant impact on the stability and reactivity of molecules. For example, a molecule with a lot of steric strain is likely to be less stable than a molecule with less strain. This is because the molecule with more strain is under greater stress, and it is more likely to undergo a chemical reaction to relieve that stress.
Steric strain can also influence the conformation of a molecule. Conformation refers to the different spatial arrangements of atoms in a molecule that can be interconverted by rotation about single bonds. A molecule will often adopt a conformation that minimizes steric strain. This is why you often see bulky groups oriented away from each other in molecules, rather than crowded together.
In summary, steric strain is a type of strain that arises when atoms or groups are forced too close together. This strain can significantly affect the stability, reactivity, and conformation of molecules.
What is torsional vs angular strain?
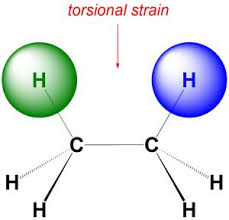
Imagine looking down one of the carbon-carbon bonds in cyclopropane. You’ll see that the hydrogen atoms on adjacent carbons are directly opposite each other. This is called an eclipsed conformation. This arrangement leads to torsional strain, which is the repulsion between electron clouds of these closely spaced atoms.
Now, think about the ideal bond angle in a molecule. For a carbon atom with four single bonds, the ideal angle is 109.5 degrees. But in cyclopropane, the bond angles are forced to be only 60 degrees. This creates angular strain, also known as ring strain, because the molecule is being squeezed into a smaller, less comfortable shape.
So, cyclopropane has both torsional strain from its eclipsed conformation and angular strain from its compressed bond angles. This combination makes cyclopropane a very unstable molecule, which is why it is highly reactive.
Let’s get a little deeper into these concepts:
Torsional strain, sometimes called dihedral strain, arises when groups connected by a single bond are eclipsed or partially eclipsed. Think of it like trying to force your arms to be perfectly aligned and held close to your body – it feels unnatural and uncomfortable! Atoms in an eclipsed conformation experience a similar discomfort as their electron clouds repel each other. In cyclopropane, this torsional strain contributes to its overall instability.
Angular strain is a type of strain that arises when the bond angles within a molecule are distorted from their ideal values. Remember, atoms want to be as far apart from each other as possible to minimize the repulsion between their electron clouds. This is why we see specific bond angles in different molecules. For example, in methane, the bond angles are 109.5 degrees. In cyclopropane, though, the bond angles are forced to be 60 degrees. This makes the molecule significantly less stable.
So, the smaller the ring, the more angular strain it experiences. You can imagine this like trying to squeeze a balloon into a smaller and smaller box. The balloon will become increasingly distorted and unstable as you squeeze it. The same thing happens with cyclopropane, where the bond angles are forced to be much smaller than their ideal value.
Understanding these types of strain can help us predict the reactivity and stability of various organic molecules. So, the next time you encounter a cyclic compound, remember to consider both torsional strain and angular strain to predict its properties and behavior.
Which has more torsional strain?
Let’s break down why this is:
Torsional strain, also known as dihedral strain, arises from the repulsion between electron clouds of bonds that are eclipsed. Imagine the bonds are like spokes on a wheel, and when they are perfectly aligned, they create the most strain. This is the eclipsed conformation. In the staggered conformation, the bonds are offset, minimizing this repulsion.
Think of it like this: When you have two people standing really close together, they are likely to bump into each other more often. This is like the eclipsed conformation, where the bonds are close and have a lot of strain. In the staggered conformation, the people are spaced out, and they have less of a chance of bumping into each other.
The difference in energy between the eclipsed and staggered conformations is called the torsional energy barrier. This barrier is what determines how easily a molecule can rotate around its single bond. For example, ethane has a torsional energy barrier of about 3 kcal/mol. This means that it takes 3 kcal/mol of energy to rotate the ethane molecule from a staggered conformation to an eclipsed conformation.
The torsional strain is a key factor in determining the conformation of molecules. It can have a significant impact on the properties of molecules, such as their reactivity, boiling point, and melting point.
See more here: What Is Torsional Strain? | Torsional Strain Vs Steric Strain
What is steric strain?
It’s important to understand steric strain when you’re trying to figure out how a molecule is shaped and how its shape affects the way it interacts with other molecules. For instance, if a molecule is really crowded, it might be harder for it to react with other molecules or it might even make the molecule less stable. This can be especially important in the field of chemistry, where scientists are always trying to design molecules with specific properties.
Steric strain can be influenced by several factors. One of the main factors is the size of the atoms or groups of atoms involved. Think of it like trying to fit a big couch into a small room—it’s going to be a lot more difficult than trying to fit a small armchair. The bigger the atoms or groups of atoms, the more likely they are to cause steric strain.
Another factor that affects steric strain is the shape of the molecule. If the molecule is arranged in a way that makes the atoms or groups of atoms close together, there’s going to be more steric strain. This is why understanding molecular shapes is crucial to understanding steric strain.
Steric strain isn’t always a bad thing, though. In some cases, it can actually be used to our advantage. For example, steric strain can be used to make certain reactions happen faster or to make molecules more stable. It’s all about understanding the underlying forces at play and how to manipulate them for our own purposes.
What is torsional strain in chemistry?
Now, you might be wondering why this is important. Well, torsional strain plays a big role in figuring out the most stable shapes of organic molecules. You see, molecules want to be as comfy and relaxed as possible, and that means minimizing this torsional strain. Think of it like trying to find the best position to sit in a chair – you want to avoid those awkward, strained positions, right? The same goes for molecules!
Torsional strain is also a key factor in understanding conformational isomerism, which is the idea that molecules can have different shapes even though they have the same atoms connected in the same order. For example, think about ethane. It has two methyl groups that can rotate around the carbon-carbon single bond. When those methyl groups are directly opposite each other, it’s called the anti conformation, which has the lowest torsional strain. But when they’re twisted, like in the gauche conformation, there’s more torsional strain because the electron clouds of the methyl groups bump into each other.
So, torsional strain is a fundamental concept in chemistry that helps us understand how molecules arrange themselves in space and what makes some arrangements more stable than others. It’s all about those tiny interactions between electrons, and understanding them gives us a deeper insight into the world of molecules!
What is the difference between steric hindrance and torsional strain?
Think of steric hindrance as a broad term. It happens when atoms or groups are close to each other, making the molecule less stable due to the repulsion between their electron clouds.
Torsional strain, however, is a more specific type of strain that occurs when atoms or groups are eclipsed.
Imagine spinning a propeller. When the blades are lined up, they create the most resistance (like a torsional strain). This resistance is due to the interaction of electron clouds and the overlap of their orbitals.
Steric hindrance can occur in different conformations, not just eclipsed ones. Think of it as a general crowding issue.
Torsional strain is specifically about the energy penalty associated with the eclipsing interaction of atoms or groups, which is the most extreme form of steric hindrance.
Here’s an analogy: Imagine trying to squeeze into a crowded elevator. The feeling of being squished and uncomfortable represents steric hindrance. If you’re standing directly in front of someone, that’s like torsional strain – the most extreme form of that crowding.
While steric hindrance is a broader concept that can affect a molecule’s overall stability, torsional strain is a more specific type of strain that arises from eclipsed conformations.
What are the three types of steric and angular strain?
Angular strain happens when the bond angles in a molecule are different from the ideal angles. Think of it like a bent straw – it takes more energy to keep it bent than it does to keep it straight. This strain occurs when atoms are forced to bond at angles that are less than or greater than the optimal angle for that type of bond. For example, in cyclopropane, a three-membered ring, the bond angles are 60 degrees, which is significantly less than the ideal 109.5 degrees for a tetrahedral carbon. This causes the molecule to be strained and less stable.
Torsional strain, also known as dihedral strain, arises from the repulsion between electron clouds on atoms that are connected by a single bond. Imagine holding a string of beads in your hand. When you rotate one bead, you might bump into the next bead. Likewise, when atoms are rotated around a bond, the electron clouds on adjacent atoms can interact, causing instability. This strain is minimized when the atoms are in staggered conformations where the electron clouds are as far apart as possible. In contrast, eclipsed conformations, where the electron clouds are aligned with each other, lead to higher torsional strain.
Steric strain, also known as van der Waals strain, occurs when non-bonded atoms or groups within a molecule get too close to each other. It’s like trying to squeeze too many people onto a small bus – things get uncomfortable! This strain is a result of the repulsive forces between electron clouds of atoms or groups that are close together. In general, bulky groups create more steric strain. For example, in a molecule like tert-butane, the three methyl groups are bulky and create steric strain.
These three types of strain play a crucial role in determining the overall shape, stability, and reactivity of molecules. Understanding them helps us to predict and explain the behavior of molecules in chemical reactions and to design new molecules with specific properties.
See more new information: musicbykatie.com
Torsional Strain Vs Steric Strain: Understanding The Differences
Hey there, chemistry enthusiasts! Today, we’re diving into the fascinating world of torsional strain and steric strain, two major players in the game of molecular stability. You might be wondering, “What’s the difference between these two strains anyway?” Well, let’s break it down.
Imagine you’re building a model with Legos. You want to connect two bricks, but the design forces them into an awkward, twisted position. That’s kind of like torsional strain. It’s the strain that arises when bonds are rotated out of their ideal staggered conformation, which is like the most comfortable position for the bricks.
Think about it this way:
Staggered conformation: The LEGO bricks are perfectly aligned, no awkward angles, everything is smooth sailing.
Eclipsed conformation: The bricks are forced to twist, putting stress on the connections, making it less stable.
Now, imagine you’re trying to squeeze too many LEGO bricks into a small space. You’re bumping them against each other, causing friction and making the whole structure unstable. That’s steric strain. It occurs when atoms or groups of atoms get too close to each other, causing repulsion due to their electron clouds.
Think of it this way:
Steric strain: Imagine you’re trying to fit a giant LEGO castle into a tiny box. The pieces are just too big, and they’re bumping into each other. That’s steric strain.
No steric strain: Imagine having a spacious room to build your LEGO castle. The pieces can be spread out, and you have plenty of room to move them around.
Torsional Strain: The Twist
Let’s zoom in on torsional strain. It’s all about the dihedral angle, which is basically the angle between two planes formed by atoms. In a staggered conformation, the dihedral angle is 60°, and the bonds are far apart, resulting in minimal strain. However, in an eclipsed conformation, the dihedral angle is 0°, the bonds are right on top of each other, and you get that uncomfortable twisted feeling.
The culprit here is the electron-electron repulsion between the bonds, which creates instability.
Steric Strain: The Bump
Next up, we have steric strain. It happens when atoms or groups of atoms get too close, like when you’re trying to squeeze too many Legos into a small space. The electron clouds of these atoms start bumping into each other, creating a repulsive force that destabilizes the molecule.
The bigger the atoms or groups, the more likely they are to experience steric strain. Think of bulky groups like tert-butyl or isopropyl as those big, bulky LEGO pieces that are difficult to fit together.
Real-World Applications
These concepts are not just theoretical. They have real-world implications in organic chemistry. Understanding torsional strain and steric strain helps us predict the shape and stability of molecules, which are critical in understanding how chemicals react and behave.
For example, torsional strain influences the conformation of cycloalkanes. You’ll find that cyclohexane exists in a chair conformation to minimize torsional strain, while cyclobutane prefers a puckered conformation to minimize steric strain.
Similarly, steric strain plays a major role in determining the reactivity of molecules. Bulky groups can hinder reactions by blocking access to reactive sites. This is why steric hindrance is a key factor in organic reactions.
FAQs
1. What are the key differences between torsional strain and steric strain?
Torsional strain: Occurs due to the rotation of bonds around a single bond, leading to electron repulsion between adjacent bonds.
Steric strain: Arises from the repulsion between non-bonded atoms or groups due to their close proximity.
2. How can I identify the presence of torsional or steric strain in a molecule?
Torsional strain: Look for eclipsed conformations, where bonds are directly aligned.
Steric strain: Look for bulky groups that are close to each other, leading to non-bonded interactions.
3. What are some examples of molecules that experience torsional and steric strain?
Torsional strain: Cycloalkanes like cyclobutane and cyclopentane.
Steric strain: Molecules with bulky groups like tert-butyl or isopropyl.
4. What are the consequences of torsional and steric strain?
Torsional strain: Reduced stability of the molecule and increased energy.
Steric strain: Can hinder reactions, decrease reactivity, and affect the physical properties of the molecule.
5. How can I minimize torsional and steric strain?
Torsional strain: Choose the staggered conformation for the single bond, where bonds are furthest apart.
Steric strain: Choose molecular conformations where bulky groups are as far apart as possible.
And there you have it – a deep dive into the world of torsional strain and steric strain! Understanding these concepts will help you gain a deeper appreciation for the intricate world of molecules and their behavior.
What is the difference between steric strain and
Steric strain exists only in molecules who have four or more bonds, since steric strain is defined as the repulsion felt between atoms at four or more bonds separated from each other forced closer than their Chemistry Stack Exchange
Torsional Strain and Steric Strain – YouTube
Torsional Strain and Steric Strain. This organic chemistry video tutorial provides a basic introduction into torsional strain and steric strain using Newman YouTube
3.4. Types of Strain in Molecules – Introduction to
Learn the difference between torsional strain and steric strain, two types of strain that molecules may experience due to electronic repulsion. Torsional strain occurs when electron clouds from two atoms separated by three OPENPRESS.USASK.CA
Torsional and Steric Strain – Chemistry Steps
Learn how to identify and calculate the energy of torsional and steric strain in Newman projections of alkanes and cycloalkanes. See examples, Chemistry Steps
What is the difference between steric and torsional strain?
We talk about torsional strain mainly when we draw Newman projection in the case of Eclipsed and Staggered. Steric strain is higher in the case of eclipsed Socratic
Understanding Torsional and Steric Strain in Chemistry
Learn the definitions, causes, and effects of torsional and steric strain, two types of energy associated with molecular rotation and spatial arrangement. Explore how mathematics warreninstitute.org
8.2: Conformational Analysis – Chemistry LibreTexts
b) Torsional strain – Tendency of s-bonds to rotate in order to acquire a more stable conformation. c) Angle strain – Increase in potential energy due to bond angles being forced to depart from ideal values in Chemistry LibreTexts
Steric Strain in Molecular Organics – Yang – Wiley Online Library
Torsional strain and steric strain play significant roles in strain energy for the substituted groups on two adjacent atoms. Torsional strain is the energy to counteract Wiley Online Library
Conformational analysis of butane (video) | Khan Academy
So there’s torsional strain there, but there’s also steric strain, or steric hindrance, which we saw in the video. These two methyl groups, the hydrogens can Khan Academy
Torsional Strain And Steric Strain
Strain In Organic Molecules | Angle Or Baeyer Strain | Steric Strain | Torsional Or Pitzer Strain
What Is Torsional Strain?
Explain Different Types Of Strain In Molecules | Stereochemistry | Organic Chemistry
Staggered, Eclipsed, Anti, And Gauche Conformers
Stability Of Cycloalkanes – Angle Strain
Newman Projections
[Tối Ưu Hóa Trong Thiết Kế Cơ Khí]Bài 01: Véctơ Gradient Và Ma Trận Hessian
Link to this article: torsional strain vs steric strain.
See more articles in the same category here: https://musicbykatie.com/wiki-how/
