Table of Contents
Why do you need to calibrate a spectroscope?
Calibration ensures the instrument is functioning correctly and that there are no defects affecting the data. These defects could be anything from a misaligned component to a faulty sensor. By calibrating the spectrometer, you can be confident that the data you collect is reliable and accurate.
Let’s break it down a little further. Imagine you are trying to identify a specific chemical in a sample. When you shine light through the sample and analyze it with a spectrometer, you’re essentially looking for a unique fingerprint – a pattern of light absorption and emission that corresponds to that chemical. Now, if your spectrometer isn’t calibrated correctly, those fingerprints might be distorted, making it difficult to identify the chemical accurately. This is where calibration comes in. It essentially acts as a “tune-up” for your spectrometer, ensuring it provides a clear and accurate representation of the light passing through the sample.
Think of it like this: if you’re trying to tune a guitar, you need to make sure each string is vibrating at the correct frequency. Similarly, a spectrometer needs to be calibrated so that it can accurately measure the wavelengths of light passing through it.
By calibrating your spectrometer, you ensure that the wavelengths of light are measured correctly, allowing you to obtain accurate data about the sample you’re analyzing.
Why does the spectrometer need to be calibrated before measuring an absorbance?
Imagine you’re baking a cake, but your measuring cups are all wonky! You might end up with a cake that’s too dry or too wet, and it won’t taste right. The same goes for a spectrometer. If it’s not calibrated correctly, it won’t give us accurate measurements of the light absorbed by a sample. This could lead to inaccurate results in our experiments or analysis.
Think of calibration like setting a baseline for the instrument. We use a standard solution with a known absorbance to “teach” the spectrometer what a specific absorbance value should look like. Once the instrument knows this baseline, it can then compare all other samples to it and give us accurate readings.
Think of it like a ruler. You need to know the length of one inch to measure other objects. The standard solution is our “one inch” and the spectrometer is our “ruler.” We calibrate to establish this baseline so that the instrument can accurately “measure” absorbance.
What do you need to calibrate the spectrophotometer?
First, navigate to the Analyze Light page in your spectrophotometer software. On the bottom toolbar, you’ll find a Calibrate Wavelengths button. Click this button to initiate the calibration process.
A Wavelength Calibration slider will appear in the Tools menu on the left side of your screen. Now, gently insert the Fiber Optic Cable into the cuvette holder of your spectrometer. The cable will act as a reference point for the calibration procedure.
Understanding Spectrophotometer Calibration
Calibrating your spectrophotometer is crucial for obtaining accurate and reliable results. The process involves adjusting the instrument’s wavelength scale to ensure that it aligns with known reference wavelengths. This is achieved by using a specific light source, like the Fiber Optic Cable, that emits a known wavelength.
During calibration, the spectrophotometer compares the light intensity detected by the instrument to the known wavelength of the light source. Any discrepancies are then corrected, adjusting the instrument’s wavelength scale to match the reference values.
Think of it like tuning a musical instrument. Just as a guitar needs to be tuned to play accurate notes, your spectrophotometer needs to be calibrated to produce accurate measurements. By calibrating your instrument, you ensure that the wavelengths it detects are precisely aligned with the actual wavelengths of the light source.
Why is Calibration Important?
Accurate calibration is paramount for various reasons. First, it ensures that your spectrophotometer measures wavelengths correctly, leading to precise absorbance and transmittance readings.
Second, proper calibration eliminates potential errors caused by slight drifts in the instrument’s wavelength scale over time. These drifts can occur due to factors like temperature changes, aging components, or even minor physical movement.
By regularly calibrating your spectrophotometer, you minimize the impact of these factors, ensuring consistent and reliable results for your experiments.
Why should wavelength scale be calibrated?
Spectrometers and other wavelength-sensitive instruments can drift over time due to environmental factors. Think about it like a finely tuned instrument that gets a little out of whack with temperature changes or vibrations. This drift can cause your readings to be off, making your data less accurate. Regular wavelength calibration ensures your instrument stays in tune, delivering the precise measurements you need. It’s like giving your instrument a check-up, making sure it’s performing at its best.
Let’s dive a little deeper into why calibration is so essential. When you calibrate your instrument, you’re essentially creating a reference point for its wavelength scale. You’re telling the instrument exactly where each wavelength should be. Think of it like setting a ruler to zero before you start measuring something.
There are a few different ways to calibrate a wavelength scale. One common method is to use a known light source with well-defined spectral lines. This source is like a “ruler” for the instrument. By comparing the measured spectral lines to the known positions, the instrument’s wavelength scale can be adjusted for accuracy.
Calibration is a critical step in ensuring the quality of your spectroscopic measurements. By keeping your instrument calibrated, you can have confidence in the accuracy and reliability of your data. This means you’ll get the most out of your instrument and avoid potential errors that could affect your research or analysis.
Why is calibration necessary?
Think about it this way: Imagine you’re baking a cake. You follow the recipe carefully, but your measuring cups and spoons are off. The cake might not rise properly, or it could be too sweet or too dry. The same principle applies to just about everything we use – from the cars we drive to the medications we take. When measurements are inaccurate, the results can be disastrous.
Calibration helps to eliminate these risks by ensuring that measuring instruments are working correctly. Regular calibration helps guarantee that the instruments are providing precise and reliable measurements, which is critical for maintaining consistency, improving efficiency, and protecting safety. Whether it’s a simple thermometer or a complex piece of scientific equipment, calibration plays a vital role in ensuring that we can trust the data we’re getting.
Why are calibration curves necessary in spectroscopy?
Now, when we measure the absorbance of an unknown sample, we can use the calibration curve to determine its concentration. We simply find the absorbance of our unknown sample on the y-axis of the calibration curve and then follow a line down to the x-axis to find the corresponding concentration.
Let’s break this down a little further:
Spectroscopy is a technique that uses light to identify and quantify different substances. In spectroscopy, we shine a beam of light through a sample and measure the amount of light that passes through it.
Absorbance is a measure of how much light is absorbed by the sample. The more light a sample absorbs, the higher its absorbance will be.
Standards are solutions with known concentrations of the analyte we are trying to measure.
Calibration curves help us to relate the absorbance of a sample to its concentration.
Think of it like this: If you have a recipe for a cake, but you don’t know how much flour you need, you could use a calibration curve to help you out. You would make a few cakes using different amounts of flour (your standards) and then measure how big each cake is (your absorbance). You would then plot the results on a graph (your calibration curve) to show the relationship between the amount of flour and the size of the cake. Once you have your calibration curve, you could use it to determine the amount of flour you need to make a cake of a specific size.
Calibration curves are essential in spectroscopy because they provide a way to accurately and reliably determine the concentration of a sample. They are used in a wide variety of applications, including environmental monitoring, food safety, and medical diagnostics.
Why is it important to calibrate a spectrophotometer with a blank?
Think of it like this: Imagine you’re trying to measure the amount of sugar in a cup of coffee. If you just pour the coffee directly into the spectrophotometer, the instrument will also detect the color of the coffee, which can affect the measurement. To get an accurate reading, you need to first measure the color of the coffee without any sugar (the blank). Then, you subtract this background reading from the reading you get when you add the sugar. This gives you a precise measurement of just the sugar.
By using a blank, you are essentially telling the spectrophotometer what a “zero” value looks like. This allows the instrument to accurately measure the absorbance of the compound you are interested in, without being influenced by other components in the solution.
Here’s another way to think about it: Imagine you’re trying to weigh a piece of fruit. If you weigh the fruit on a scale that’s already set to zero, you’ll get an accurate weight. But if the scale is already showing a weight because it hasn’t been calibrated to zero, you’ll get a reading that includes the weight of the scale itself. In the same way, the blank sets the spectrophotometer to zero, ensuring you get an accurate measurement of your compound of interest.
See more here: Why Does The Spectrometer Need To Be Calibrated Before Measuring An Absorbance? | Why Would A Spectroscope Need To Be Calibrated
Why should a spectrophotometer be calibrated?
Wavelength calibration, in particular, is crucial because it ensures the spectrophotometer can pinpoint and measure the exact light wavelengths you’re interested in. This is essential for getting accurate readings of the substance you’re analyzing. By regularly calibrating your spectrophotometer, you can be confident that your results are consistent and reliable, which is important for research, quality control, or any other application where accuracy is key.
Let’s dive a little deeper into why wavelength calibration is so important. When you’re using a spectrophotometer, you’re essentially shining light through a sample and measuring how much light passes through it. This information can then be used to identify the sample or quantify its concentration.
However, over time, the spectrophotometer’s ability to accurately measure the wavelengths of light can become compromised. This is because the components within the spectrophotometer responsible for selecting the desired wavelengths can shift slightly due to factors like temperature changes, vibrations, or general wear and tear. This shift in wavelength accuracy can lead to inaccurate measurements and, in turn, incorrect conclusions about your sample.
Wavelength calibration helps ensure that the spectrophotometer is accurately measuring the correct wavelengths. This is usually done by measuring the absorbance of a known standard solution at specific wavelengths. By comparing the measured absorbance values to the known values for the standard, the spectrophotometer can be adjusted to ensure it’s reading the wavelengths correctly.
By regularly calibrating your spectrophotometer, especially its wavelength accuracy, you can be confident that your results are reliable and trustworthy. This can save you time and effort in the long run by avoiding the need to repeat experiments due to inaccurate measurements. Plus, it helps to ensure that your results are scientifically sound and can be confidently used for research, quality control, or any other important applications.
How do you calibrate a spectroscope?
To calibrate, you’ll create a calibration plot. Think of it as a cheat sheet for your spectroscope. You’ll plot the scale reading you get from your experiment on the x-axis and the known wavelength value (found in a reference book or online) on the y-axis. You can use a full sheet of paper to make sure your plot is clear and easy to read. This calibration plot will be unique to each spectroscope.
Why is this important? Well, your spectroscope might not be perfectly aligned. The scale might be slightly off, meaning the wavelength you read might not be totally accurate. The calibration plot helps you correct for this!
You can use the calibration plot to find the corrected wavelength of any light source, like the hydrogen spectrum you might be measuring. Imagine you’re measuring a light source and get a reading of 50 on your spectroscope’s scale. You find the point on your calibration plot corresponding to 50 on the x-axis, then read the corresponding wavelength value on the y-axis. That’s your corrected wavelength!
Now, let’s dive a little deeper into creating this calibration plot.
You’ll need a light source with known wavelengths. For example, you could use a mercury lamp or a neon lamp. These lamps emit light at specific, well-defined wavelengths. You can find these wavelengths in a reference book or online.
Next, you’ll shine the light from your chosen lamp through the spectroscope and observe the resulting spectrum. The light will be spread out, and you’ll see bright lines at specific locations on the spectroscope’s scale. Note down the scale readings for each of these lines.
Now, you have two sets of data: the scale readings you obtained from your experiment and the known wavelengths from your reference source. Plot this data on your graph. You can use a ruler and pencil or even a spreadsheet program to help you create a clean and accurate plot.
Finally, you can use this calibration plot to correct the wavelengths you measure for other light sources! It’s a handy tool that helps ensure your spectroscopic measurements are as accurate as possible.
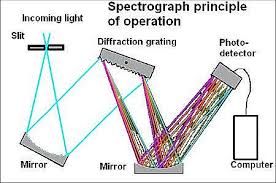
How does a spectroscope work?
At one end of the spectroscope, you’ll find a diffraction grating. This is a special film made of plastic, but with a twist! Thousands of tiny grooves are etched onto its surface. When light shines through this grating, it gets bent and spread out into its different wavelengths. Each wavelength corresponds to a specific color. Think of it like separating white light into its rainbow colors.
Here’s the cool part: The diffraction grating is like a tiny prism, but it’s much more efficient at separating light. You can see this when you look through the spectroscope. You’ll see a rainbow of colors, each representing a different wavelength of light. The light source you’re viewing determines the colors you see.
If you’re looking at a white light bulb, you’ll see all the colors of the rainbow. If you’re looking at a red LED, you’ll only see red light. The arrangement of colors tells you a lot about the light source itself.
To understand how the diffraction grating works, let’s imagine you’re walking through a forest and you come across two trees. When you look at the trees from far away, they seem to be close together, but as you get closer, you can tell they’re separate trees. The same thing happens with light waves when they pass through the diffraction grating. The grooves act like the trees, creating a separation between the different wavelengths of light. The tighter the grooves are, the more light gets separated.
So there you have it – the diffraction grating is the key ingredient that makes a spectroscope work! It’s like a mini-prism that separates light into its constituent colors, allowing us to study the secrets of the universe, one wavelength at a time.
Why do we need a spectroscope?
Here’s how it works: When light passes through a prism, it gets separated into its different colors, creating a spectrum. Each element in the light source emits specific wavelengths of light, which appear as bright lines in the spectrum. These lines are like fingerprints, uniquely identifying the element. This is because each element has a unique set of energy levels, and when an electron moves from one level to another, it emits light at a specific wavelength.
By analyzing the spectrum, a spectroscope can identify the elements present in the light source. This information can be used to determine if the lights are truly fluorescent or if there are any other elements present, such as mercury or sodium. It can also be used to measure the intensity of the light emitted, which can be useful for determining the efficiency of the light source.
In short, a spectroscope is an indispensable tool for identifying and analyzing light sources, making it an invaluable tool in various fields, from astronomy to industry.
See more new information: musicbykatie.com
Spectroscope Calibration: Why It’S Essential
Think of it this way: A spectroscope breaks down light into its different colors, like a rainbow. This helps us identify the chemical makeup of a substance, kind of like a fingerprint. But if the spectroscope isn’t calibrated properly, the “fingerprint” will be messed up, and you won’t be able to get accurate information.
So, why do we calibrate? There are a few key reasons:
To ensure accuracy: Like any measuring device, a spectroscope can drift over time. This means that the wavelengths it measures might not be exactly correct. Calibration corrects these errors, making sure you get precise measurements. Imagine you’re a chemist analyzing a sample. If the spectroscope is off, you could end up with the wrong results, leading to incorrect conclusions.
To maintain consistency: Calibration ensures that the spectroscope consistently produces the same results under different conditions. This is important if you’re comparing data from different experiments or measurements.
To meet standards: Many scientific and industrial applications require that instruments meet certain standards for accuracy. Calibration helps you meet those requirements. It’s like having a stamp of approval that says your spectroscope is reliable and provides accurate results.
How does calibration work?
Calibration involves comparing the spectroscope’s readings to a known standard. Think of it as checking your spectroscope against a “gold standard” to see how close it is.
Here’s a simplified explanation:
1. Reference material: We use a sample with a known spectrum, kind of like a reference guide for the spectroscope.
2. Calibration procedure: We shine light through the reference material and record its spectrum using the spectroscope.
3. Comparison: We compare the recorded spectrum with the known spectrum of the reference material. If there’s any difference, we adjust the spectroscope’s settings to align it with the known standard.
Different types of calibration
There are different ways to calibrate a spectroscope, depending on its type and intended use. Some common methods include:
Wavelength calibration: This involves adjusting the spectroscope to ensure it accurately measures the wavelengths of light. You’re essentially making sure the rainbow is in the right order and the colors are in the correct positions.
Intensity calibration: This corrects for variations in the intensity of the light being measured. Imagine you’re trying to measure the brightness of a star. If your spectroscope doesn’t account for the distance of the star, you might get inaccurate readings. Intensity calibration adjusts for such factors.
Baseline correction: This is used to remove any background noise or interference in the spectrum. Think of it as removing any static or fuzz from a recording to get a clearer signal.
Calibration frequency
How often you calibrate your spectroscope depends on how frequently you use it and the level of accuracy required. But as a general rule, regular calibration is always a good idea. Here’s a quick guide:
Frequent use: Calibrate regularly, even daily, especially if you’re performing sensitive measurements.
Less frequent use: Calibrate at least every few months or before a critical experiment.
Environmental factors: If the spectroscope is exposed to extreme temperatures, humidity, or vibrations, calibrate more frequently.
Benefits of calibration
Accurate results: Calibration ensures that your spectroscope provides precise and reliable measurements, leading to better data analysis and interpretation.
Improved reproducibility: Consistent calibration helps you reproduce experiments and obtain similar results over time. This is crucial for research and development.
Compliance with standards: Calibrated instruments meet regulatory and industry standards, allowing you to use your spectroscope for applications where accuracy is paramount.
Increased confidence: Knowing that your spectroscope is calibrated gives you confidence in your measurements, allowing you to make informed decisions based on reliable data.
FAQs
Q: Can I calibrate a spectroscope myself?
A: Yes, you can calibrate some spectroscopes yourself, but it’s usually best to consult the manufacturer’s instructions or have it calibrated by a qualified technician. Calibrating a spectroscope requires specific knowledge and equipment.
Q: What are the risks of not calibrating a spectroscope?
A: Not calibrating your spectroscope can lead to inaccurate results, incorrect interpretations, and potentially wasted time and resources. It can also affect the validity of your findings, making it difficult to compare your results with others or publish them.
Q: How can I tell if my spectroscope needs to be calibrated?
A: If you notice any significant deviations in your results, or if your spectroscope has been exposed to harsh environments, it’s a good idea to have it calibrated. You can also check the manufacturer’s recommendations for calibration intervals.
Q: What is the difference between wavelength calibration and intensity calibration?
A: Wavelength calibration ensures that the spectroscope accurately measures the wavelengths of light. Intensity calibration corrects for variations in the intensity of the light being measured.
Q: Does the type of spectroscope matter when it comes to calibration?
A: Yes, the type of spectroscope and its intended use will influence the calibration method and frequency. Some spectroscopes have automatic calibration features, while others require manual adjustments.
Q: Can I calibrate a spectroscope without a reference material?
A: No, you need a reference material with a known spectrum to calibrate a spectroscope. This serves as a standard for comparison.
Q: Can I use a different reference material than the one recommended by the manufacturer?
A: It’s best to use the reference material recommended by the manufacturer, as it’s specifically designed for that particular spectroscope. Using a different reference material might not produce accurate results.
Q: Is it necessary to calibrate a spectroscope for every measurement?
A: Not necessarily. But it’s always a good idea to check the calibration periodically, especially if you’re working with critical applications or if the spectroscope has been exposed to harsh environments.
Q: What are some common sources of error in spectroscope measurements?
A: Common sources of error include misalignment, drift, environmental factors, and improper operation. Calibration helps to minimize these errors.
Q: Can I use a calibrated spectroscope for any application?
A: While calibration helps improve the accuracy of a spectroscope, it’s important to choose the right instrument for your specific application. Different spectroscopes have different capabilities and limitations.
Calibrating the Spectroscope: Visible Light Spectroscopy
Calibrating the Spectroscope: The calibration of your spectroscope is necessary to correct for systematic error. This is done by comparing your experimentally- determined wavelengths to wavelengths obtained from the literature. A convenient source of uiuc.edu
Spectrophotometer Calibration and Validation: Ensuring Accuracy
Why Calibration and Validation of Spectrophotometers are Important? 1. Ensuring Accuracy. Spectrophotometers measure the amount of light absorbed by a Drawell
Lab #7: Analyzing Light: The Spectroscope
A spectroscope is similar to a prism in that it can break up light into its component. At one end of the spectroscope is a square film of material that acts like a prism. The film is called a diffraction grating. It is Chemistry Land
2.1.5: Spectrophotometry – Chemistry LibreTexts
You need a spectrometer to produce a variety of wavelengths because different compounds absorb best at different wavelengths. For example, p-nitrophenol Chemistry LibreTexts
experimental physics – How is a spectrometer is calibrated for …
For the case of spectrophotometers, we can easily calibrate the device (intensity wise) taking the light source inside the device as the reference, without any Physics Stack Exchange
Hand-Held Spectroscope: Calibrating the Spectroscope – ChemEd X
Readings obtained from a hand-held spectroscope may not correlate precisely with known values measured with more sophisticated instruments. Therefore, calibrating the Chemical Education Xchange
The Importance of Spectrometer Calibration – XRF
When a spectrometer is calibrated, it ensures the instrument is ‘set back to zero’ and that no defects are present. Any defects will impact the reliability and accuracy of the data XRF Scientific
AAVSO Guide to Getting Started in Spectroscopy
Section 1 gives an overview of the design and principles of spectrographs, and briefly discusses the equipment required to produce a fully calibrated set of spectroscopic AAVSO
114 Procedures for Wavelength Calibration and Spectral
The wavelength cal-ibration of the instrument is performed by scanning through the grating angles and measuring a spectrum with known wavelengths. A comparison of the meas NIST
How Does A Spectrophotometer Work?
Spectroscope Calibration
Calibrating A Spectrometer
Calibrating And Using The Spectrophotometer
How Does A Spectrometer Work?
Calibrating Your I-Phos Spectrometer
Excel Tutorial 2 Spectroscope Calibration
Uv-Vis Spectrometer Calibration [Spectrasuite]
Link to this article: why would a spectroscope need to be calibrated.
See more articles in the same category here: https://musicbykatie.com/wiki-how/
