Table of Contents
What is the principle of Salkowski reaction for cholesterol?
Here’s a breakdown of the reaction:
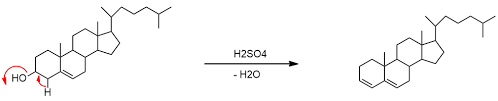
1. Cholesterol, when treated with concentrated sulfuric acid, undergoes dehydration to form cholestadienes, which are unsaturated hydrocarbons.
2. These cholestadienes then react with acetic anhydride, leading to the formation of colored products. The color of the solution changes from a pale yellow to a blue-green hue, confirming the presence of cholesterol.
The intensity of the color produced is directly proportional to the concentration of cholesterol present in the sample. This allows for a semi-quantitative estimation of cholesterol levels.
It’s important to note that the Salkowski reaction is a relatively simple and quick test, but it’s not as accurate as more sophisticated analytical methods like gas chromatography or mass spectrometry. However, it remains a valuable tool for screening purposes and can be useful in situations where more advanced techniques are not readily available.
How does the Salkowski test work?
Let’s break down exactly what’s happening in the test:
The Sample: The terpenoid containing sample is often an extract obtained from a plant or other natural source.
Chloroform: Chloroform acts as a solvent to help dissolve the terpenoids and make them readily available for the reaction.
Sulfuric Acid: This strong acid is the key to the color change reaction. When terpenoids come into contact with concentrated sulfuric acid, they undergo a chemical transformation, resulting in the formation of colored compounds.
The reddish-brown color observed at the interface is a direct result of this chemical reaction. The intensity of the color can sometimes be used to estimate the concentration of terpenoids present in the sample.
Here’s a little more about terpenoids:
Terpenoids are a large and diverse class of organic compounds found in plants, animals, and even some microorganisms. They’re responsible for the aromas and flavors of many essential oils and contribute to the defense mechanisms of plants. Examples include limonene found in citrus fruits, camphor from camphor trees, and beta-caryophyllene, a major component of black pepper.
The Salkowski test is widely used in various fields, including:
Pharmacology: To identify and quantify terpenoids in medicinal plants.
Food Chemistry: To analyze the composition of essential oils used in food products.
Cosmetics: To assess the quality and authenticity of essential oils used in skincare and fragrance products.
It’s worth noting that while the Salkowski test is a good starting point for detecting terpenoids, further analysis may be necessary to confirm their identity and quantify their concentration. More advanced techniques like chromatography and spectroscopy are often used for this purpose.
What are the results of the Salkowski test for lipids?
When a solution containing lipids is mixed with the Salkowski reagent (a solution of concentrated sulfuric acid and chloroform), a distinct color change occurs. This change indicates the presence of lipids, confirming a positive result.
Here’s what you’ll see in a positive Salkowski test:
The upper layer (chloroform) turns bluish-red to violet. This color change is due to the reaction between the sulfuric acid and the cholesterol in the sample.
The lower layer (sulfuric acid) turns yellow to green, sometimes with a greenish glow. This color change is also due to the reaction between the sulfuric acid and the cholesterol, but in this case, the sulfuric acid is acting as an oxidizing agent.
How does the Salkowski test work?
The Salkowski test relies on the reaction between concentrated sulfuric acid and cholesterol. When these two substances react, they form a series of colored compounds that are responsible for the characteristic color changes observed in a positive test.
The bluish-red to violet color in the chloroform layer is caused by the formation of a complex called “cholestadienyl sulfate”. This complex is formed when the sulfuric acid reacts with the double bond in the cholesterol molecule.
The yellow to green color in the sulfuric acid layer is caused by the formation of other colored compounds, including “cholestadienyl sulfate” and “cholestadienyl sulfonate”. These compounds are formed through a series of oxidation reactions.
Important notes:
* The intensity of the color change is proportional to the concentration of cholesterol in the sample.
* The Salkowski test is not specific for cholesterol, and other sterols can also produce a positive result.
* The Salkowski test is not quantitative, meaning it does not tell you how much cholesterol is present.
While the Salkowski test is a useful tool for detecting lipids, it is not a definitive test. Other tests, such as gas chromatography and mass spectrometry, are needed to confirm the presence and identify the specific type of lipids in a sample.
What is the conclusion of Salkowski test for cholesterol?
The red color is due to the formation of a chromophore during the reaction. This chromophore is a molecule that absorbs light in the visible spectrum, resulting in the red color. The intensity of the red color is directly proportional to the concentration of cholesterol in the solution.
However, it is important to note that the Salkowski test is not a specific test for cholesterol. Other steroids can also produce a similar color change. Therefore, this test is only a preliminary test and should be confirmed by other more specific analytical methods.
What is the Salkowski reaction?
Here’s how it works: You take a sample containing cholesterol and dissolve it in chloroform. Then, you carefully add concentrated sulfuric acid to the solution. If cholesterol is present, you’ll see two distinct layers form. The top layer, which is the chloroform layer, will turn a beautiful red to violet color. At the same time, the bottom layer, the sulfuric acid layer, will glow a greenish hue.
This color change is due to a chemical reaction called dehydration, where the sulfuric acid removes water molecules from the cholesterol molecule. This forms a new compound called cholestadiene, which is responsible for the characteristic colors observed in the Salkowski reaction.
Let’s break down the chemistry a little further. The sulfuric acid acts as a strong dehydrating agent, pulling water molecules (H2O) from the cholesterol molecule. This dehydration process results in the formation of a double bond within the cholesterol molecule, leading to the creation of cholestadiene. The newly formed double bond, along with the specific arrangement of atoms in cholestadiene, is what gives rise to the absorption of certain wavelengths of light, making the chloroform layer appear red to violet. The greenish glow observed in the sulfuric acid layer is likely due to the formation of other reaction byproducts, which can absorb and emit light in the green region of the electromagnetic spectrum.
The Salkowski reaction is a relatively simple yet effective test for detecting cholesterol. It has been widely used in laboratories for many years, providing a quick and reliable way to identify the presence of this important lipid in various samples.
Which colour does cholesterol produced in Salkowski test?
Let me explain this in more detail. The Salkowski test involves mixing a sample of cholesterol with concentrated sulfuric acid and chloroform. The reaction produces a colored layer in the chloroform, and this layer’s color can tell us whether cholesterol is present.
The exact color of the chloroform layer depends on the type of cholesterol present and the reaction conditions. However, a violet or purple color is a strong indication that cholesterol is present. This color comes from the formation of oxidation products of cholesterol. When cholesterol is exposed to air, it can be oxidized, and these oxidation products can then react with sulfuric acid to produce the characteristic violet or purple color.
You can think of it like this: When cholesterol is exposed to air, it’s like leaving an apple out on the counter. The apple will eventually turn brown because it’s oxidizing. In the same way, cholesterol can oxidize in the presence of air, and these oxidation products are responsible for the violet or purple color in the Salkowski test.
So, if you see a violet or purple color in the chloroform layer during the Salkowski test, it’s a good sign that cholesterol is present.
What is the reagent of Salkowski test?
Let’s break down the components and their roles in the reagent:
Ferric chloride (FeCl3): This is a chemical compound that acts as an oxidizing agent. It reacts with cholesterol, leading to the formation of colored products that are indicative of its presence.
Distilled water: Distilled water plays a crucial role in diluting the concentrated sulfuric acid and ensuring the proper concentration of the ferric chloride solution.
Concentrated sulfuric acid (H2SO4): This is the most crucial component of the reagent. It acts as a dehydrating agent, causing the cholesterol molecule to undergo a series of chemical reactions. These reactions result in the formation of colored products that can be visually observed and quantified.
The specific proportions of these components in the reagent are important for ensuring the accuracy and reliability of the Salkowski test. By carefully controlling the concentration of each component, the reaction between the reagent and cholesterol can be optimized, leading to a clear and distinct color change that is easily detectable.
The Salkowski test is a valuable tool for detecting cholesterol in various samples, including blood serum, tissue extracts, and other biological fluids. The color change observed in the test is directly proportional to the amount of cholesterol present in the sample.
It’s important to note that the Salkowski reagent should be handled with caution as concentrated sulfuric acid is corrosive. Proper safety precautions should always be taken when working with this reagent.
See more here: How Does The Salkowski Test Work? | Salkowski Test For Cholesterol Mechanism
What is The Salkowski reaction of cholesterol?
Here’s how it works: You take a sample of cholesterol and dissolve it in chloroform. Then, you carefully add concentrated sulfuric acid. If cholesterol is present, something magical happens! You’ll see two distinct layers form. The top layer, which is the chloroform layer, will turn a beautiful red to violet color. At the same time, the bottom layer, the sulfuric acid layer, will glow a striking green. It’s like a little chemical magic show!
So, why does this happen? The sulfuric acid acts as a strong dehydrating agent and oxidizer. It reacts with the cholesterol molecule, causing it to break down and form new compounds. These compounds are responsible for the distinctive color changes. The red-violet color in the chloroform layer is due to the formation of a colored complex, while the green glow in the sulfuric acid layer comes from the oxidation products.
The Salkowski reaction is a good example of how chemical reactions can produce visually striking results. It’s a simple test that can be used to identify cholesterol, and it’s a fun demonstration of the principles of organic chemistry.
How does Salkowski’s test detect cholesterol?
Let’s break down how this colorimetric reaction works:
Sulfuric acid is a strong acid that acts as a dehydrating agent, removing water molecules from the cholesterol molecule. This process forms a cholesterol derivative known as cholestadienyl which is highly reactive and forms colored compounds in the presence of acetic anhydride.
Acetic anhydride is an organic compound that reacts with the cholestadienyl to form a colored complex. The color of this complex varies depending on the amount of cholesterol present.
Colorimetry is used to measure the intensity of this color, which allows us to determine the concentration of cholesterol in the sample. This means that the darker the color, the higher the cholesterol concentration.
The Salkowski’s test provides a quick and easy way to detect the presence of cholesterol in a sample. It is commonly used in laboratories for research and clinical purposes.
What is Salkowski’s test?
Let’s break down how Salkowski’s test works and why it’s important. This test, also known as the Salkowski reaction, is a chemical test that detects the presence of cholesterol in a sample of blood or other bodily fluids. The test relies on the reaction between cholesterol and a specific reagent, which causes a color change. The intensity of the color change is directly proportional to the amount of cholesterol present in the sample.
Here’s a simplified explanation:
Step 1: The Sample: A small sample of blood is taken from the patient.
Step 2: The Reagent: The blood sample is mixed with a specific chemical reagent.
Step 3: The Reaction: The cholesterol in the blood sample reacts with the reagent, causing a color change. The color change will be more intense if there’s more cholesterol.
Step 4: The Results: A lab technician measures the intensity of the color change and uses this information to determine the amount of cholesterol in the sample.
The results of Salkowski’s test are then used to assess a patient’s cholesterol levels and help doctors make informed decisions about their treatment plan. If the test reveals high cholesterol levels, it might lead to lifestyle changes, medication adjustments, or further investigations to pinpoint the underlying cause of the elevated cholesterol.
What is a Salkowski biochemical test?
The Salkowski test is a specific type of colorimetric test that relies on the interaction of a chemical reagent with the substance being tested. The Salkowski test is particularly useful for detecting the presence of cholesterol and other sterols in a sample. The test involves the use of a specific reagent that reacts with sterols to produce a characteristic color change. The intensity of the color change is directly proportional to the concentration of sterols in the sample. This reaction is based on the ability of sterols to react with concentrated sulfuric acid, which results in the formation of colored compounds. The Salkowski test is relatively simple to perform and can be conducted in a laboratory setting.
It’s important to note that the Salkowski test is a qualitative test, meaning it indicates the presence or absence of a substance. It doesn’t provide a precise measurement of the concentration of the substance being tested. While it’s been a valuable tool in the past, it has largely been replaced by more modern and precise analytical techniques.
The Salkowski test has a long history in biochemistry and has played a significant role in the development of our understanding of various biological processes. While it may not be as widely used today as it once was, the legacy of Ernst Leopold Salkowski and his contributions to the field of biochemistry remain important and continue to inspire scientists today.
See more new information: musicbykatie.com
Salkowski Test For Cholesterol: Mechanism And Applications
Alright, let’s dive into the fascinating world of cholesterol testing, specifically the Salkowski test. You might be wondering why this test is so important, and how it helps doctors understand your cholesterol levels. Well, let’s break it down.
The Importance of Cholesterol
Firstly, it’s important to understand that cholesterol isn’t inherently bad. It’s a waxy, fat-like substance that’s essential for building healthy cells, producing hormones, and absorbing vitamins. The problem arises when we have too much of it, especially the “bad” kind, known as low-density lipoprotein (LDL). High LDL levels can clog arteries, increasing the risk of heart disease and stroke. On the flip side, the “good” cholesterol, high-density lipoprotein (HDL), helps remove LDL from the bloodstream, reducing the risk of heart disease.
The Salkowski Test: A Key to Understanding Cholesterol
Now, let’s talk about the Salkowski test. This test is a simple, colorimetric method used to determine the presence of cholesterol in a sample. It relies on the reaction of cholesterol with concentrated sulfuric acid, producing a distinct color change. The intensity of the color is directly proportional to the concentration of cholesterol in the sample.
How the Salkowski Test Works
Here’s the breakdown of the Salkowski test mechanism:
1. Sample Preparation: A small amount of the sample, containing cholesterol, is mixed with a solution of chloroform. Chloroform acts as a solvent, dissolving the cholesterol and allowing it to react with the acid.
2. Reaction with Sulfuric Acid: Concentrated sulfuric acid is carefully added to the chloroform solution. The sulfuric acid is a strong oxidizing agent, causing a chemical reaction with the cholesterol molecules. This reaction results in the formation of colored products.
3. Colorimetric Observation: The color change observed is crucial for determining the presence and concentration of cholesterol. The resulting colors range from pale yellow to bright red, with the intensity of the color indicating the level of cholesterol in the sample.
Advantages of the Salkowski Test
The Salkowski test boasts several advantages:
Simplicity: It’s a relatively straightforward and easy-to-perform test, making it accessible in various settings.
Cost-Effectiveness: It’s a cost-effective method for detecting cholesterol, compared to other more sophisticated techniques.
Speed: The results can be obtained quickly, providing a rapid assessment of cholesterol levels.
Limitations of the Salkowski Test
While the Salkowski test has its benefits, it also has limitations:
Specificity: It’s not entirely specific for cholesterol and may react with other substances that produce similar color changes. This can lead to false-positive results.
Accuracy: Its accuracy can be affected by factors such as the concentration of sulfuric acid, the temperature of the reaction, and the presence of other interfering substances in the sample.
Quantitative Analysis: It’s primarily a qualitative test, indicating the presence or absence of cholesterol. It doesn’t provide precise quantitative data on the exact amount of cholesterol present.
Interpreting the Salkowski Test Results
Now, let’s talk about interpreting the results of the Salkowski test. Here’s how it breaks down:
Positive Result: A positive result is indicated by a color change in the solution, ranging from pale yellow to bright red. The intensity of the color indicates the level of cholesterol present. A darker color signifies a higher concentration of cholesterol.
Negative Result: A negative result is indicated by the absence of a color change. This means there’s no detectable cholesterol in the sample.
Understanding the Salkowski Test Results
While the Salkowski test can be a useful tool for screening, it’s important to remember that it’s not a definitive diagnostic test. If you’re concerned about your cholesterol levels, consult a healthcare professional. They can perform more comprehensive tests, such as a lipid panel, which provides a more detailed picture of your cholesterol profile.
The Importance of a Lipid Panel
A lipid panel is a blood test that measures different types of lipids, including:
Total Cholesterol: The total amount of cholesterol in your blood.
LDL Cholesterol: The “bad” cholesterol.
HDL Cholesterol: The “good” cholesterol.
Triglycerides: A type of fat found in your blood.
A lipid panel provides a more complete understanding of your cholesterol levels and helps your doctor assess your risk of heart disease.
Taking Action: Lowering Cholesterol
If your cholesterol levels are high, your doctor may recommend lifestyle changes, such as:
Healthy Diet: Focus on consuming a diet rich in fruits, vegetables, whole grains, and lean protein.
Regular Exercise: Aim for at least 30 minutes of moderate-intensity exercise most days of the week.
Weight Management: Maintaining a healthy weight can help lower cholesterol levels.
Quit Smoking: Smoking damages blood vessels and increases the risk of heart disease.
When to Seek Medical Attention
If you experience any of the following symptoms, consult a healthcare professional immediately:
* Chest pain or pressure
* Shortness of breath
* Dizziness or lightheadedness
* Nausea or vomiting
* Numbness or tingling in your arms or legs
* Sudden weakness or loss of coordination
FAQs: Addressing Your Questions
Here are some common questions about the Salkowski test and cholesterol:
1. What are the units for measuring cholesterol?
Cholesterol levels are typically measured in milligrams per deciliter (mg/dL).
2. What is the normal range for cholesterol?
The normal range for total cholesterol is generally less than 200 mg/dL. The target LDL cholesterol level is less than 100 mg/dL.
3. What are some foods high in cholesterol?
Foods high in saturated and trans fats tend to raise cholesterol levels. These include:
* Fatty meats (like red meat and processed meats)
* Full-fat dairy products (like butter and whole milk)
* Fried foods
* Processed foods
* Some baked goods
4. Is cholesterol hereditary?
Yes, cholesterol levels can be influenced by genetics. If you have a family history of high cholesterol, it’s important to get your cholesterol levels checked regularly.
5. What are the long-term consequences of high cholesterol?
High cholesterol can lead to various health problems, including:
Heart disease: High LDL cholesterol can build up in your arteries, leading to plaque formation and narrowing of the arteries. This can increase your risk of heart attack, stroke, and other cardiovascular problems.
Stroke: If a blood clot forms in an artery that has been narrowed by plaque, it can block the flow of blood to the brain, causing a stroke.
Peripheral artery disease: High cholesterol can also affect the arteries in your legs and feet, leading to peripheral artery disease.
6. Can I get my cholesterol levels checked at home?
There are home cholesterol testing kits available, but they’re not as accurate as lab tests. It’s best to consult a healthcare professional for accurate and reliable cholesterol testing.
7. What can I do to lower my cholesterol naturally?
There are several things you can do to lower your cholesterol naturally:
Eat a healthy diet: Choose fruits, vegetables, whole grains, and lean proteins. Limit saturated and trans fats, cholesterol-rich foods, and added sugars.
Exercise regularly: Aim for at least 30 minutes of moderate-intensity exercise most days of the week.
Maintain a healthy weight: If you’re overweight or obese, losing even a small amount of weight can help lower your cholesterol levels.
Quit smoking: Smoking damages blood vessels and increases the risk of heart disease.
8. How often should I get my cholesterol checked?
The frequency of cholesterol testing depends on your age, risk factors, and current cholesterol levels. Your doctor can recommend a suitable testing schedule.
9. What medications can be used to lower cholesterol?
If lifestyle changes aren’t enough to lower your cholesterol levels, your doctor may prescribe medications like statins, which block the production of cholesterol in the liver.
Wrapping it Up
Understanding your cholesterol levels is crucial for maintaining good health. The Salkowski test offers a simple and cost-effective way to screen for the presence of cholesterol. However, it’s important to remember that it’s not a definitive diagnostic test. If you’re concerned about your cholesterol levels, consult your doctor for comprehensive testing and personalized recommendations. Take charge of your heart health, and remember, knowledge is power!
What is the mechanism of Salkowski reaction of
Cholesterol in chloroform is treated with concentrated sulfuric acid. A positive test exhibits two distinct layers, the upper Chemistry Stack Exchange
Salkowski test for cholesterol – Its principle and procedure
Salkowski’s test, based on the principles of colorimetry, is an essential technique for detecting cholesterol in various biological samples. Its simplicity and reliability make it a medicalstudyhub.com
Salkowski test for cholesterol – Its principle and
Salkowski test for cholesterol: Salkowski test is used to detect cholesterol in a solution. This test is named after a German biochemist Ernst Leopold Salkowski. Medical Study Zone
Salkowski’s test: Part 1 (Identification of Cholesterol) – YouTube
Imbalance in lipid metabolism can lead to major clinical problems such as obesity and atherosclerosis. …more. Principle: Cholesterol as a derived lipid is a member of the group YouTube
Salkowski test – Oxford Reference
A test for cholesterol. When concentrated sulfuric acid is added to a chloroform solution of cholesterol, the chloroform layer shows a red to blue colour and the acid layer shows … Oxford Reference
Salkowski test, 1 of the best method for total cholesterol
Salkowski test is the best method for the estimation of the total cholesterol in the given sample. Salkowski test uses the salkowski reagent; a coloring reagent infobiochem.com
Analytical reviews in clinical biochemistry: the quantitative
Early mechanistic studies on colour production by the action of acids on cholesterol have been reviewed by Kritchevesky.f It was suggested that the mechanism of the sulphuric SAGE Journals
Lipids – Wiley Online Library
We studied the reactivity of cholesterol under LB conditions and provide definitive NMR characterization for approximately 20 products, whose structure and AOCS Publications
Salkowski’s Reagent Test as a Primary Screening Index for …
Salkowski’s reagent test, which is often used in detect-ing indolic substances. Among 69 isolates grown in a low-nitrogen medium supplemented with L-tryptophan (TRP), culture Taylor & Francis Online
Salkowski’S Test: Part 1 (Identification Of Cholesterol)
Salkowski’S Test: Part 2 (Identification Of Cholesterol)
Salkowski’S Test, Qualitative Test For Cholesterol, Ospe Question, Viva Questions
Salkowski’S Test For Cholesterol With Demo
Cholesterol Test Procedure | Cholesterol Reagent | Procedure For Performing Cholesterol Reagent Test
Salkowski Test For Cholesterol Practical Biochemistry By Dr. Rashid
Qualitative Test Of Cholesterol(Salkowskis Test ; Liebermann-Burchard Test)In English Language
Salkowski Test Performance
Link to this article: salkowski test for cholesterol mechanism.
See more articles in the same category here: https://musicbykatie.com/wiki-how/
