Table of Contents
What is the reduction of camphor NaBH4?
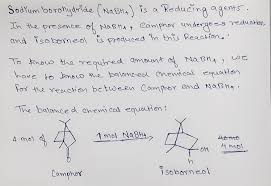
You’re curious about the reduction of camphor with NaBH4, and you’ve stumbled upon the balanced equation:
4C10H16O + NaBH4 + 4H2O → 4C10H18O + NaB(OH)4
This equation tells us that four moles of camphor react with one mole of NaBH4 in the presence of four moles of water to produce four moles of borneol (the reduced form of camphor) and one mole of sodium tetrahydroborate.
But how do we use this equation to understand the actual reaction process? Let’s break it down step by step.
First, we need to determine the moles of camphor we’re working with. Let’s say we’re using 0.25 grams of camphor. The molar mass of camphor is 152.26 g/mol. To calculate the moles of camphor, we divide the mass by the molar mass:
0.25 g / 152.26 g/mol = 0.0016 moles of camphor
Next, we need to determine the moles of NaBH4 required for this reaction. Let’s assume we’re also using 0.25 grams of NaBH4. The molar mass of NaBH4 is 37.83 g/mol. So, the moles of NaBH4 are:
0.25 g / 37.83 g/mol = 0.0066 moles of NaBH4
Remember, according to the balanced equation, four moles of camphor react with one mole of NaBH4. This means that our 0.0016 moles of camphor would require only 0.0004 moles of NaBH4 for complete reaction. Since we have more NaBH4 than needed, we can say that NaBH4 is in excess in this scenario.
Why is NaBH4 in excess important? Well, it ensures that all the camphor molecules get reduced to borneol. If we had less NaBH4, some of the camphor might remain unreacted.
This is just a basic overview of the camphor reduction reaction. There are many other factors that can influence the outcome of this reaction, such as the solvent used, the reaction temperature, and the reaction time.
How does sodium borohydride reduce ketone of camphor?
The mechanism of sodium borohydride reduction of a ketone or aldehyde is very similar to that of hydroboration of a C=C bond. The first step is the addition of the hydride ion (H-) to the carbonyl carbon of the ketone or aldehyde. This results in the formation of an alkoxide ion. The alkoxide ion is then protonated by water to form the alcohol. This reaction is often called a nucleophilic addition reaction.
Sodium borohydride is a very mild reducing agent and is typically used in reactions that are carried out at room temperature. Sodium borohydride is also a very selective reducing agent and will not reduce other functional groups, such as alkenes or alkynes.
Let’s take a closer look at how sodium borohydride reduces a ketone or aldehyde. The first step in the mechanism is the formation of a complex between the ketone or aldehyde and sodium borohydride. This complex is formed by the interaction of the lone pair of electrons on the oxygen atom of the carbonyl group with the boron atom of sodium borohydride. The next step is the transfer of a hydride ion from sodium borohydride to the carbonyl carbon of the ketone or aldehyde. This step is often called a nucleophilic addition reaction. The result of this reaction is the formation of an alkoxide ion. The alkoxide ion is then protonated by water to form the alcohol.
Sodium borohydride is a very versatile reagent that can be used to reduce a variety of ketones and aldehydes. For example, sodium borohydride can be used to reduce camphor to isoborneol.
Camphor is a ketone that is found in the essential oil of the camphor tree. It is a white, crystalline solid that has a strong, penetrating odor. Camphor is used in a variety of applications, including as a moth repellent, an antiseptic, and a flavoring agent.
Isoborneol is an alcohol that is also found in the essential oil of the camphor tree. It is a white, crystalline solid that has a mild, camphoraceous odor. Isoborneol is used in a variety of applications, including as a fragrance ingredient and a pharmaceutical ingredient.
Sodium borohydride is a very effective reagent for reducing ketones and aldehydes to alcohols. This reaction is a very important reaction in organic chemistry and is used to synthesize a variety of important compounds.
What is the product of borohydride reduction of camphor?
Let’s break down the process. Camphor, a naturally occurring compound found in plants, boasts a unique structure with a carbonyl group (C=O). Sodium borohydride acts as a reducing agent, delivering a hydride ion (H-) to this carbonyl group. This addition of a hydride ion transforms the carbonyl group into a hydroxyl group (OH), effectively reducing the camphor molecule. However, the reduction can happen in two different ways, leading to the formation of two isomers: borneol and isoborneol.
The difference lies in the stereochemistry of the newly formed hydroxyl group. Borneol has the hydroxyl group in an *endo* position, meaning it’s on the same side as the bridgehead carbon, while isoborneol has the hydroxyl group in an *exo* position, meaning it’s on the opposite side of the bridgehead carbon.
This difference in stereochemistry arises due to the way the hydride ion attacks the carbonyl group. Sodium borohydride can approach from either side of the molecule, leading to two possible products. These two isomers, borneol and isoborneol, have distinct physical and chemical properties, offering a glimpse into the fascinating world of stereoisomers.
Why use sodium borohydride in reduction?
The solvents used in a sodium borohydride reduction reaction are important because they influence the reactivity of the reagent. For example, if you want to reduce an aldehyde or ketone, you’ll typically use a polar protic solvent like methanol or ethanol. These solvents help to stabilize the intermediate formed during the reduction process. However, if you’re working with a more sensitive functional group, you might need to use a less polar solvent like tetrahydrofuran (THF) or diethyl ether.
Here’s a more detailed breakdown of why sodium borohydride is a popular choice for reductions:
Selectivity: Sodium borohydride is selective for carbonyl compounds, meaning it won’t reduce other functional groups like esters, amides, or nitro groups. This selectivity is crucial for reactions where you need to control the outcome and avoid unwanted side products.
Mildness: Sodium borohydride is a mild reducing agent, meaning it doesn’t require harsh conditions like high temperatures or pressures. This makes it safer and easier to work with compared to other reducing agents like lithium aluminum hydride.
Versatility: Sodium borohydride can be used in a variety of solvents, making it adaptable to different reaction conditions. It can also be used in the presence of various other functional groups, further enhancing its versatility.
Stability: Sodium borohydride is stable in the solid state and can be stored for long periods without significant degradation. This makes it a convenient and reliable reagent for organic synthesis.
In summary, sodium borohydride is a valuable tool for organic chemists. Its selectivity, mildness, versatility, and stability make it an excellent choice for reducing aldehydes, ketones, and acid chlorides in the presence of other easily reducible functional groups.
What is the major product in the reduction of camphor?
Now, here’s the interesting part. Camphor has a bulky structure, and the hydride prefers to attack the less crowded side of the carbonyl group. This less crowded side leads to the formation of isoborneol as the major product. The other possible product, borneol, is formed in smaller amounts because the hydride has to overcome more steric hindrance to attack the more crowded side.
Let’s break it down further:
Steric factors: The bulky structure of camphor influences the hydride’s attack. It’s like trying to squeeze through a crowded doorway – the less crowded path is easier to navigate.
Electronic factors: The carbonyl group in camphor has a partial positive charge on the carbon atom and a partial negative charge on the oxygen atom. The hydride, being negatively charged, is attracted to the positive carbon atom. However, the hydride also needs to consider the steric factors.
In the end, the combination of steric and electronic factors favors the formation of isoborneol as the major product in the reduction of camphor using sodium borohydride. It’s a beautiful example of how the structure and reactivity of molecules play a crucial role in determining the outcome of chemical reactions.
Why is methanol used in reduction of camphor?
Here’s a bit more about why methanol is so helpful in this reaction:
Solubility: The ability of methanol to dissolve both camphor and sodium borohydride is crucial. This ensures that the reactants are in close contact, allowing the reduction to happen efficiently.
Polarity:Sodium borohydride is a strong reducing agent, and its high polarity means it needs a solvent that can interact with it effectively. Methanol, being a polar solvent, can do this, while diethyl ether, a non-polar solvent, can’t.
Boiling Point: After the reaction is finished, you need to separate the product from the reaction mixture. Methanol’s higher boiling point makes it easier to evaporate it off, leaving behind the reduced product, camphor.
Hydrolysis: During the reduction process, a tetraalkylborate intermediate is formed. Methanol’s higher boiling point helps to facilitate the hydrolysis of this intermediate, releasing the final product.
In essence, methanol’s unique properties make it an excellent choice as a solvent for the reduction of camphor. It allows the reaction to proceed smoothly and makes it easy to isolate the desired product.
How does NaBH4 reduce ketones?
1) Nucleophilic addition: The hydride ion (H-) from NaBH4 attacks the electrophilic carbon atom of the ketone. This forms an alkoxide intermediate.
2) Protonation: The alkoxide intermediate then reacts with a proton source (like water or methanol) to form the secondary alcohol.
NaBH4 is a mild reducing agent, meaning it only reduces certain functional groups. While it readily reduces ketones to secondary alcohols, it won’t reduce esters or amides under normal conditions.
Let’s delve deeper into how NaBH4 interacts with ketones.
The hydride ion (H-) in NaBH4 acts as a powerful nucleophile. This means it’s attracted to positively charged areas in molecules. Ketones have a partially positive carbon atom due to the electron-withdrawing nature of the oxygen atom. This makes the carbon atom an excellent target for the hydride attack.
When NaBH4 is added to a ketone solution, the hydride ion attacks the carbon atom. This forms an alkoxide intermediate, where the oxygen atom now has a negative charge.
This alkoxide intermediate is unstable. To stabilize, it needs to regain a proton. This is where the proton source comes in. A proton source like water or methanol will donate a proton to the negatively charged oxygen atom, forming the final product: a secondary alcohol.
The reaction with NaBH4 is highly selective. It prefers to react with ketones and aldehydes, leaving esters and amides untouched. This makes NaBH4 a valuable tool for organic chemists, allowing them to selectively reduce specific functional groups in a molecule.
See more here: How Does Sodium Borohydride Reduce Ketone Of Camphor? | Reduction Of Camphor With Sodium Borohydride
How does sodium borohydride reduce camphor?
Sodium borohydride is a powerful reducing agent commonly used in organic chemistry. When it reacts with camphor, it produces two isomers: borneol and isoborneol.
This reaction is shown in Scheme 1: Reduction of Camphor.
You’ll see that in a methanol solvent, the sodium borohydride attacks the bottom side of the camphor structure. This is the less sterically hindered side, meaning it’s easier for the sodium borohydride to access and reduce the carbonyl group.
Now, let’s delve deeper into the mechanism:
The Reduction Process
1. Nucleophilic Attack: The hydride ion (H-) from sodium borohydride acts as a nucleophile, attacking the electrophilic carbon of the carbonyl group in camphor. This attack creates a new bond between the carbon and the hydride ion.
2. Protonation: The resulting alkoxide ion is then protonated by a molecule of methanol, yielding the alcohol product.
Stereochemistry: The Formation of Borneol and Isoborneol
The reason we get two isomers, borneol and isoborneol, is due to the stereochemistry of the reaction. Camphor has a rigid structure with a defined shape. This means that the hydride attack can occur from two different directions:
– Borneol: The hydride attack occurs from the endo side of the camphor molecule, leading to the formation of borneol.
– Isoborneol: The hydride attack occurs from the exo side of the camphor molecule, leading to the formation of isoborneol.
The endo and exo terms refer to the relative positions of the hydroxyl group (OH) in the final product. In borneol, the hydroxyl group is on the same side as the bridgehead carbon atoms, while in isoborneol, it is on the opposite side.
Factors Influencing the Ratio of Products
The ratio of borneol and isoborneol formed depends on several factors, including:
– Solvent: The nature of the solvent used can influence the rate of attack from different sides of the camphor molecule.
– Temperature: The reaction temperature can also affect the product ratio.
This is a simplified explanation of how sodium borohydride reduces camphor. It’s a fascinating example of how a simple reagent can lead to the formation of multiple products based on the stereochemistry of the reaction.
How does isoborneol reduce camphor?
Isoborneol is a compound that arises from the reduction of camphor. This reduction process involves a chemical reaction where the carbonyl group in camphor is transformed into an alcohol group.
Sodium borohydride is a powerful reducing agent that does this magic. Imagine camphor as a molecule with a carbonyl group (C=O) sticking out. The sodium borohydride acts like a tiny helper, attacking the carbonyl group and changing it into an alcohol group (C-OH).
Now, camphor has a unique structure with two sides – one is bulky and the other is less crowded. The sodium borohydride prefers to attack the less crowded side, giving us isoborneol. The process involves the addition of water which completes the formation of the alcohol group.
You might be wondering, “Why two isomers?” Well, there’s a slight twist! When sodium borohydride attacks the carbonyl group in camphor, it can create two different arrangements of atoms – these are called isomers. We call one of these isoborneol, and the other, borneol. The difference between the two lies in the position of the methyl group on the molecule.
The reduction of camphor with sodium borohydride in methanol is a classic example of stereochemistry – the study of the three-dimensional arrangement of atoms in molecules. This reaction showcases how subtle differences in the chemical environment can lead to the formation of different isomers.
Here’s a simple breakdown:
Camphor is the starting material with a carbonyl group.
Sodium borohydride is the reducing agent.
Methanol is the solvent.
Isoborneol is the primary product, formed by the attack of sodium borohydride on the less sterically hindered side of camphor.
Borneol is the other isomer, formed by the attack on the more sterically hindered side.
Understanding this reaction is essential for chemists and anyone interested in the fascinating world of organic reactions. It’s a perfect example of how simple changes can create a whole new set of molecules!
What is the reducing agent for camphor?
Sodium borohydride (NaBH4) is a common reducing agent used to convert camphor into a mixture of borneol and isoborneol. This reaction is a classic example of a hydride reduction where the hydride ion (H-) from NaBH4 attacks the carbonyl group of camphor, resulting in the formation of the alcohol products.
While methanol is often used as a solvent for this reaction, it’s considered toxic. The good news is that ethanol, a non-toxic alternative, can also be used as a solvent.
Here’s the breakdown:
Camphor has a carbonyl group (C=O) which is reduced to a hydroxyl group (C-OH) to form borneol and isoborneol.
NaBH4 provides the hydride ion (H-) necessary for the reduction.
Methanol and ethanol act as solvents, helping the reaction proceed smoothly.
The key takeaway: NaBH4 is the reducing agent that makes this reaction happen, while methanol and ethanol are solvents.
Now, let’s dive a bit deeper:
The reduction of camphor to borneol and isoborneol is a stereoselective reaction, meaning that the reaction favors the formation of a particular stereoisomer. In this case, the reaction produces a mixture of borneol and isoborneol because the carbonyl group in camphor can be attacked from either side of the molecule.
Borneol and isoborneol are both chiral molecules, meaning they have a non-superimposable mirror image. This difference in their structure leads to different properties. Borneol is a crystalline solid with a strong camphoraceous odor, while isoborneol is an oily liquid with a milder odor.
The choice of solvent can affect the ratio of borneol to isoborneol produced. For example, using methanol as a solvent generally leads to a higher yield of isoborneol, while using ethanol can result in a higher yield of borneol.
So, there you have it – a breakdown of the reducing agent and solvents involved in the conversion of camphor to borneol and isoborneol.
See more new information: musicbykatie.com
Reduction Of Camphor With Sodium Borohydride | What Is The Reduction Of Camphor Nabh4?
Let me break it down for you.
What’s happening here?
Imagine camphor as a mountain. It has a pointy peak, a carbonyl group (C=O). Sodium borohydride is our trusty mountain climber, and it’s going to reduce that peak, transforming it into a more gentle slope, a hydroxyl group (OH).
The Chemistry Behind it
Sodium borohydride (NaBH4) is a powerful reducing agent. It loves to donate hydride ions (H-) to compounds with electrophilic centers, like the carbonyl group in camphor.
Camphor (C10H16O) is a bicyclic ketone. It’s the “mountain” with that carbonyl peak we’re talking about.
The Reaction
The reaction happens in a couple of steps:
1. Hydride attack: The hydride ion from sodium borohydride attacks the carbonyl group in camphor. This breaks the double bond between carbon and oxygen and forms a new bond between the carbon and the hydride ion.
2. Protonation: The resulting intermediate is then protonated by a proton source (usually water or a weak acid). This adds a hydrogen atom to the oxygen, creating the hydroxyl group, and you’ve got your borneol!
Let’s Get Practical
Materials you’ll need:
Camphor: You can find this in pharmacies or online.
Sodium borohydride: This can be purchased from chemical supply companies.
Methanol: This is a common solvent in organic chemistry.
Water: We’ll need some water for workup.
Ice bath: This will help keep the reaction temperature low.
Beaker or flask: For the reaction to take place.
Stirring device: To mix the reaction mixture.
Filter paper: To separate the product.
Drying agent: Like anhydrous magnesium sulfate, to remove any water from the product.
Safety Precautions
Sodium borohydride is reactive and can react violently with water. Always handle it with care.
Methanol is flammable. Make sure you work in a well-ventilated area and keep it away from heat and open flames.
Wear gloves and eye protection to protect yourself from any potential splashes or spills.
Procedure
1. Dissolve your camphor: Start by dissolving a certain amount of camphor in methanol in a beaker or flask.
2. Add sodium borohydride: Carefully add the sodium borohydride to the solution in small portions while stirring. Make sure the temperature stays below 25 °C. An ice bath can help with this.
3. Reaction time: Allow the reaction to continue for a few hours or overnight, depending on the temperature and the amount of reactants you used.
4. Work-up: Carefully add water to the reaction mixture to destroy any leftover sodium borohydride. You might notice a slight gas evolution. Don’t worry, that’s normal.
5. Extract the borneol: Use a separating funnel to extract the borneol from the mixture. You’ll likely get a white solid.
6. Wash and dry: Wash the solid with water and then dry it with a drying agent like anhydrous magnesium sulfate.
7. Recrystallization: Recrystallize the borneol from a suitable solvent (like hexane or toluene) to obtain a pure product.
Important Points
The reaction is exothermic, meaning it generates heat. Control the temperature carefully to prevent any unwanted side reactions.
The reaction is sensitive to air and moisture. Work quickly and carefully to avoid any problems.
Use a suitable solvent: The best solvent depends on the specific conditions of the reaction. Methanol is a popular choice.
What are the advantages of using sodium borohydride for this reduction?
Sodium borohydride offers a few key advantages over other reducing agents for this reaction:
Mildness: It’s relatively mild, so it’s less likely to cause unwanted side reactions.
Selectivity: It selectively reduces ketones and aldehydes without affecting other functional groups in the molecule.
Safety: It’s generally safe to handle and store under appropriate conditions.
That’s the basics of reducing camphor with sodium borohydride!
Let me know if you have any more questions.
Frequently Asked Questions (FAQs)
1. What is the yield of the reaction?
The yield of this reaction can vary depending on the reaction conditions, but you can usually expect a yield of 60-80%.
2. What happens if I don’t use an ice bath?
If you don’t use an ice bath, the reaction may proceed too quickly and produce unwanted byproducts.
3. Can I use another solvent besides methanol?
Yes, you can use other solvents like ethanol or isopropanol. However, the reaction rate and yield may be affected.
4. What are some alternative reducing agents?
Lithium aluminum hydride (LiAlH4) is a stronger reducing agent that can also reduce ketones. However, it’s more reactive and dangerous to handle.
Diisobutylaluminum hydride (DIBAL-H) is a milder reducing agent that can selectively reduce esters to aldehydes.
5. What is the importance of camphor reduction?
Camphor reduction to borneol is a good example of a stereoselective reaction. This means that the reaction produces a specific stereoisomer of the product, in this case, borneol.
6. How can I confirm if I have successfully synthesized borneol?
Melting point analysis: Borneol has a characteristic melting point of 204-208 °C.
Spectroscopic analysis: You can use NMR spectroscopy (nuclear magnetic resonance) to confirm the structure of borneol.
7. Can I use this reaction to synthesize other compounds?
Yes, you can use sodium borohydride to reduce other ketones and aldehydes. The reaction conditions may need to be adjusted depending on the specific compound.
8. Are there any safety concerns associated with this reaction?
As mentioned earlier, sodium borohydride is reactive and can react violently with water. Always handle it with care.
9. What are some applications of borneol?
Borneol has a number of applications, including:
Fragrances: It’s used in perfumes and other scented products.
Medicinal properties: It has been used in traditional medicine for its analgesic and anti-inflammatory properties.
Insect repellent: It is known to repel insects, especially mosquitoes.
10. Where can I learn more about organic chemistry reactions?
There are many great resources available for learning more about organic chemistry reactions:
Textbooks: There are many excellent organic chemistry textbooks that cover a wide range of topics.
Online resources: Websites like Khan Academy, Chem LibreTexts, and Organic Chemistry Portal offer free online courses and tutorials.
Videos: YouTube channels like Crash Course and Organic Chemistry Tutor provide video explanations of organic chemistry concepts.
I hope this detailed guide has been helpful. Good luck with your camphor reduction experiments!
Reduction of Camphor: Lab Experiment – Odinity
Learn how to reduce camphor into isoborneol and borneol with sodium borohydride in this lab experiment. See the experimental procedure, results, discussion, and references for this organic synthesis reaction. odinity.com
Reduction of Camphor to Borneol using Sodium Borohydride
The reduction of camphor using the reducing agent sodium borohydride resulted in the formation of two isomers, borneol and isoborneol, as shown in Scheme 1: Reduction of wpmucdn.com
Reduction of Camphor – Cerritos College
In this experiment, you will reduce camphor, a naturally occurring ketone, using sodium borohydride. Camphor is an example of a bridged bicyclic molecule: a molecule with Cerritos College
(PDF) Reaction of camphor with sodium borohydride:
Reduction of camphor to a mixture of borneol and isoborneol was performed using NaBH4 as the reducing agent under suitable conditions. Although more effective reduction was accomplished using… ResearchGate
An Oxidation-Reduction Scheme: Borneol, Camphor, Isoborneol1
REDUCTION OF CAMPHOR WITH SODIUM BOROHYDRIDE. ohydride NaBH4, are widely used in reducing carbonyl groups. Lithium aluminum hydride, for example, wvu.edu
19.3: Reductions using NaBH4, LiAlH4 – Chemistry
In the sodium borohydride reduction the methanol solvent system achieves this hydrolysis automatically. In the lithium aluminium hydride reduction water is usually added in a second step. The lithium, Chemistry LibreTexts
Chem2O06 – 1997/98 – Experiment 7 – Department of
Learn how to use sodium hypochlorite and sodium borohydride to convert borneol to camphor and isoborneol, respectively. The experiment involves microscale techniques and gas chromatography to measure the mcmaster.ca
Reduction of Camphor – Troy University
Reduction of Camphor. TUD Department of Chemistry. When camphor is reduced with metal hydride reagents such as LiAlH¢. or NaBH¢, either borneol or isoborneol can be troy.edu
9: Exp. 35B- Reduction of Camphor – Chemistry LibreTexts
The LibreTexts libraries are Powered by NICE CXone Expert and are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Chemistry LibreTexts
Reduction of Camphor to Isoborneol | Sciencing
The process of replacing a double-bonded oxygen atom with a hydrogen atom and hydroxide ion is a type of reaction known as reduction. Chemically, a ketone (camphor) may be converted into one of its Sciencing
Reduction Of Camphor With Sodium Borohydride
Reduction Of Camphor
Reduction Of Camphor Lab
Oxidation Of Isoborneol And Reduction Of Camphor Chem2050 Part Ii Reduction
Reduction Of Camphor
The Reduction Of Camphor Lab
Mechanism For The Reduction Of Camphor To Isoborneol
Reduction Of Camphor To Alcohol By Nabh4
Link to this article: reduction of camphor with sodium borohydride.
See more articles in the same category here: https://musicbykatie.com/wiki-how/
