Table of Contents
How does H2SO4 react with alkenes?
Think of it like this: The sulfuric acid acts like a sneaky little thief, snatching a hydrogen atom from the alkene and attaching itself to the carbon where the hydrogen used to be. Let’s take ethene (C2H4) as an example. When ethene reacts with sulfuric acid, it forms ethyl hydrogensulfate (CH3CH2HSO4).
The way we write the structure of ethyl hydrogensulfate is important because it shows how all the atoms are connected. It’s a bit like a molecular puzzle, and the formula helps us see how the pieces fit together.
Now, let’s dive a little deeper into this reaction. The sulfuric acid acts as an electrophile, meaning it’s looking for electrons to grab onto. The double bond in the alkene is like a juicy target for the electrophilic sulfuric acid.
This reaction is actually a two-step process. First, the sulfuric acid attacks the double bond, forming a carbocation. A carbocation is a positively charged carbon atom that’s missing an electron. This carbocation is unstable and wants to get its electron back.
In the second step, the hydrogensulfate ion (HSO4-) joins the carbocation, forming the alkyl hydrogensulfate. The final product is a stable molecule with the sulfuric acid attached to the carbon where the double bond was.
It’s important to note that the reaction with sulfuric acid is just one way that alkenes can react. Alkenes are very versatile and can participate in many different reactions. But understanding this reaction with sulfuric acid is a good starting point for exploring the reactivity of these important organic molecules.
How do alkenes react with dilute acid?
Let’s break down how this works. Water is added across the double bond of an alkene. The acid helps to break the double bond and allow water to add on. This is kind of like a domino effect – the acid gets things started, and then water jumps in and forms the alcohol.
Here’s a more detailed explanation:
1. Protonation of the alkene: The first step is the protonation of the alkene by the acid. This means that a hydrogen ion (H+) from the acid attaches to one of the carbon atoms in the double bond, forming a carbocation. A carbocation is a positively charged carbon atom. This carbocation is unstable and wants to get rid of its positive charge.
2. Nucleophilic attack: Next, a water molecule attacks the carbocation. The oxygen atom in the water molecule donates a pair of electrons to the carbocation, forming a new bond. This bond formation results in a protonated alcohol.
3. Deprotonation: Finally, the protonated alcohol loses a hydrogen ion (H+) to form the final alcohol product.
Understanding Carbocations:
Carbocations are positively charged carbon atoms. They’re super unstable and want to get rid of their positive charge ASAP! They do this by reacting with anything that has a pair of electrons to share.
Water is a perfect candidate for this role. The oxygen atom in water has two lone pairs of electrons that it’s happy to share with the carbocation, forming a new bond and creating a protonated alcohol. This makes everything much more stable.
This reaction is a classic example of electrophilic addition. The acid acts as an electrophile, which means it’s attracted to the electrons in the double bond. This attraction leads to the addition of the water molecule across the double bond, resulting in the formation of an alcohol.
What happens when ethene is treated with dil H2SO4?
Here’s a breakdown of the reaction:
Step 1: Ethene reacts with concentrated sulfuric acid to form ethyl hydrogen sulfate. This is an intermediate compound, meaning it’s formed during the reaction but isn’t the final product.
Step 2: The ethyl hydrogen sulfate is then hydrolyzed (reacted with water) to produce ethanol.
Let’s break down the terms:
Hydration: The addition of water to a molecule.
Catalyst: A substance that speeds up a chemical reaction without being consumed in the process.
Intermediate: A temporary compound formed during a reaction before the final product is formed.
Hydrolysis: A chemical reaction where a molecule is broken down by the addition of water.
The overall reaction can be represented by this equation:
CH₂=CH₂ + H₂O → CH₃CH₂OH
Here’s the key: The dilute sulfuric acid plays a crucial role in the hydrolysis step, converting ethyl hydrogen sulfate into ethanol. It’s important to remember that the reaction doesn’t happen directly with dilute sulfuric acid. It’s a multi-step process involving concentrated sulfuric acid as a catalyst followed by hydrolysis with dilute sulfuric acid.
What does dilute H2SO4 do to an alcohol?
The process of dehydration involves removing water molecules (H₂O) from the alcohol molecule. This reaction is favored by the presence of heat and concentrated sulfuric acid. The sulfuric acid acts as a catalyst, speeding up the reaction. While sulfuric acid is crucial for the dehydration process, its oxidizing properties can lead to side reactions.
When sulfuric acid acts as an oxidizing agent, it removes electrons from the alcohol molecule. This process results in the formation of carbon dioxide (CO₂) and sulfur dioxide (SO₂) gas. These gases need to be removed from the reaction mixture to obtain a pure alkene product. The formation of these gases is a side reaction that can reduce the yield of the desired alkene product.
It’s important to note that the oxidation reaction is more likely to occur when using concentrated sulfuric acid. This is because concentrated sulfuric acid is a much stronger oxidizing agent than dilute sulfuric acid. In addition, the reaction temperature can also influence the extent of oxidation. Higher temperatures tend to favor oxidation reactions.
Let’s break down the reaction:
Alcohol + H₂SO₄ (concentrated) → Alkene + H₂O + CO₂ + SO₂
In summary, dilute sulfuric acid plays a dual role in alcohol reactions. It acts as a dehydrating agent, facilitating the formation of alkenes. However, its oxidizing nature can lead to unwanted side reactions, generating carbon dioxide and sulfur dioxide. Understanding these factors is crucial for optimizing the yield of the desired alkene product and minimizing unwanted side reactions.
What happens when alkene reacts with dilute sulphuric acid?
Let’s break down this reaction a bit further. The sulfuric acid acts as a catalyst, meaning it speeds up the reaction without being consumed itself. It does this by providing a proton (H+) to the alkene. This creates a carbocation, which is a positively charged carbon atom. This carbocation is then attacked by a water molecule, resulting in the formation of an alcohol.
The type of alcohol produced depends on where the water molecule attaches to the carbocation. For example, if the water molecule attaches to the carbon that was originally part of the double bond, then a primary alcohol is formed. If it attaches to a carbon adjacent to the double bond, then a secondary alcohol is formed. Finally, if it attaches to a carbon two carbons away from the double bond, then a tertiary alcohol is formed.
Example:
Consider the reaction of propene with dilute sulfuric acid. The first step is the protonation of the double bond, forming a carbocation. Since propene is symmetrical, the carbocation can form on either carbon.
Step 1: Propene reacts with sulfuric acid to form a carbocation intermediate.
Step 2: A water molecule attacks the carbocation, forming 2-propanol.
The 2-propanol is a secondary alcohol because the hydroxyl group (-OH) is attached to a carbon that is bonded to two other carbons.
The reaction of alkenes with dilute sulfuric acid is a very useful reaction in organic chemistry. It allows us to synthesize a variety of alcohols from readily available alkenes.
Let me know if you’d like to explore the mechanism of this reaction in more detail or have any other questions.
How do alkanes react with sulfuric acid?
Let’s break down why this happens:
Alkanes are saturated hydrocarbons: This means they contain only single bonds between carbon atoms and have the maximum number of hydrogen atoms possible. This structure makes them very stable.
Sulfuric acid is a strong acid: However, its strength isn’t enough to overcome the stability of alkanes.
No reactive sites: Alkanes lack functional groups like double or triple bonds that can readily react with sulfuric acid.
Think of it like this: alkanes are like a locked box, and sulfuric acid doesn’t have the right key to open it.
However, there are a few exceptions to this general rule. Under very specific conditions, alkanes can react with sulfuric acid.
High temperatures: Heating alkanes with sulfuric acid to very high temperatures can break some of the C-H bonds, leading to reactions.
Presence of catalysts: Certain catalysts can help to break the C-H bonds in alkanes, making them more susceptible to reaction with sulfuric acid.
Specific alkanes: Some alkanes, like those with tertiary carbons (carbons bonded to three other carbons), can react with sulfuric acid at lower temperatures.
But in general, alkanes are pretty resistant to reaction with sulfuric acid. They’re happy just being the stable, unreactive compounds they are!
What happens when alkene reacts with h2o in presence of H2SO4?
This reaction is a classic example of electrophilic addition. Let’s break down the process:
1. Protonation: The first step involves the protonation of the alkene by the sulfuric acid. This means that a hydrogen ion (H⁺) from the acid attaches to one of the carbon atoms in the double bond. This forms a carbocation, which is a positively charged species.
2. Nucleophilic Attack: The carbocation is highly reactive and is susceptible to attack by a nucleophile. In this case, the nucleophile is water (H₂O). The water molecule attacks the carbocation, forming a new bond between the oxygen atom in water and the positively charged carbon atom.
3. Deprotonation: The resulting species is a protonated alcohol. This intermediate is unstable and undergoes deprotonation, losing a proton (H⁺) to regenerate the sulfuric acid and form the final product, an alcohol.
So, the overall reaction can be summarized as follows:
* An alkene reacts with concentrated sulfuric acid to form a carbocation.
* The carbocation reacts with water to form a protonated alcohol.
* The protonated alcohol loses a proton to form the final alcohol product and regenerate the sulfuric acid.
This reaction is an important industrial process for producing alcohols from alkenes. For example, ethanol (drinking alcohol) can be produced by reacting ethene with water in the presence of sulfuric acid.
Can alkenes react with strong acids?
Let’s break down what happens:
The alkene’s double bond acts as a nucleophile and attacks the hydrogen ion (H+) from the strong acid.
Simultaneously, the bond between the oxygen and hydrogen in the acid breaks heterolytically. This means that the electrons from the bond go to the oxygen atom, resulting in the formation of a water molecule (H2O).
The carbon atom that originally had the double bond now has a positive charge and becomes a carbocation. This carbocation is highly reactive and can react with other nucleophiles present in the solution.
This reaction is called an electrophilic addition reaction. It’s a fundamental reaction in organic chemistry, leading to the formation of various important compounds.
For example, the reaction of an alkene with a strong acid like sulfuric acid (H2SO4) can lead to the formation of an alcohol. In this case, the carbocation intermediate formed reacts with water, a nucleophile present in the solution, to form an alcohol.
However, the specific product formed depends on the structure of the alkene and the reaction conditions.
Here are some things to keep in mind:
The stability of the carbocation formed is crucial. A more stable carbocation is more likely to form.
The presence of other nucleophiles can influence the reaction and lead to the formation of different products.
The reaction of alkenes with strong acids is a fascinating example of how double bonds in organic molecules react with electrophiles. It’s a fundamental reaction that forms the basis for many important synthetic pathways.
See more here: How Do Alkenes React With Dilute Acid? | Reaction Of Alkene With Dilute H2So4
What happens when alkene reacts with dilute sulfuric acid?
Here’s the key point: dilute sulfuric acid acts as a catalyst in this reaction. A catalyst is like a helper; it speeds up the reaction but doesn’t get used up in the process. So, the sulfuric acid isn’t directly involved in the formation of the alcohol. It’s there to make the whole thing happen faster.
Now, how do we determine which alcohol will form? That’s where Markovnikov’s rule comes in. This rule states that the hydrogen atom from the water molecule will attach to the carbon atom in the alkene with the most hydrogen atoms already attached. In simpler terms, the hydrogen will go to the carbon that’s already the least “crowded.”
Let’s break it down with an example: imagine you have an alkene with a double bond between a carbon with two hydrogen atoms and a carbon with one hydrogen atom. When you add water, the hydrogen from the water molecule will attach to the carbon that already has two hydrogens, resulting in a secondary alcohol.
Why does Markovnikov’s rule work? It has to do with the stability of the intermediate carbocations formed during the reaction. The carbocation with more alkyl groups attached is more stable, which is why the hydrogen atom prefers to add to the carbon with fewer alkyl groups.
In summary, when you mix an alkene with dilute sulfuric acid, you get a hydration reaction. The sulfuric acid acts as a catalyst, and the product you get depends on where the water molecule attaches. Markovnikov’s rule helps us predict the product by telling us that the hydrogen atom from the water molecule will attach to the carbon with the most hydrogen atoms already attached.
Why is H2SO4 added in alkyne hydrolysis reaction?
Here’s the breakdown:
Alkyne hydrolysis is the process of breaking down an alkyne (a hydrocarbon with a carbon-carbon triple bond) using water. The reaction produces a ketone. This reaction usually requires a strong acid catalyst like H2SO4.
H2SO4 plays a crucial role in the process:
Protonation: It protonates the alkyne, making it more susceptible to attack by water. This makes the triple bond more reactive.
Stabilization: It helps stabilize the carbocation intermediate that forms during the reaction.
HgSO4 is a special catalyst that’s added to speed things up. It acts as a Lewis acid, helping to activate the alkyne and making it easier for water to add. This increases the reaction rate and improves the yield of the ketone product.
So, why is H2SO4 added in alkyne hydrolysis?
It’s all about the chemistry. H2SO4 is a strong acid that makes the alkyne more reactive. HgSO4 is a special catalyst that helps the reaction go faster. Together, they make alkyne hydrolysis a more efficient process, resulting in the formation of ketones.
Now, let’s address how you can distinguish between alkenes and alkynes using hydrolysis reactions:
Alkenes readily undergo hydrolysis with dilute sulfuric acid, producing alcohols.
Alkynes require the additional presence of HgSO4 and H2SO4 to undergo hydrolysis. They form ketones.
Therefore, by observing the reaction conditions and the product formed, you can distinguish between alkenes and alkynes. If a reaction occurs with dilute sulfuric acid alone, it’s likely an alkene. If it requires the addition of HgSO4, it’s likely an alkyne.
Are alkenes hyrolyzed by dilute sulfuric acid?
It’s true, alkenes react with dilute sulfuric acid, but alkynes don’t. This is because alkenes have a double bond, which is more reactive than the triple bond in alkynes.
The reaction of an alkene with dilute sulfuric acid is called hydration. This reaction involves the addition of water (H₂O) to the double bond, forming an alcohol.
Here’s a breakdown of what happens:
Step 1: The sulfuric acid protonates the alkene to form a carbocation. A carbocation is a species with a positively charged carbon atom.
Step 2: The carbocation is then attacked by a water molecule.
Step 3: The resulting species loses a proton to form the alcohol.
The type of alcohol formed (primary or secondary) depends on the structure of the alkene.
* If the alkene is symmetrical, the same alcohol will be formed regardless of which carbon atom the water molecule adds to.
* If the alkene is unsymmetrical, the Markovnikov rule predicts the major product. This rule states that the hydrogen atom of the water molecule will add to the carbon atom of the double bond that already has more hydrogen atoms.
Let’s dive a bit deeper into the why behind this reaction.
Imagine the double bond in an alkene as a vulnerable spot. It’s like a bridge waiting for a strong force to break it apart. Sulfuric acid, with its acidic nature, acts like that strong force. It attacks the double bond, breaking it and adding itself in.
But alkynes are tougher. They have a triple bond, making them more resistant to this kind of attack. It’s like having a triple layer of protection on a bridge – it’s much harder to break.
The hydration process with alkenes is like building a new bridge, turning a double bond into a single bond with an alcohol group attached.
So, the next time you see an alkene and sulfuric acid, think of it as a reaction waiting to happen, a bridge waiting to be strengthened!
How does sulfuric acid react with unsymmetrical alkenes?
Now, when you’re dealing with an unsymmetrical alkene, meaning the carbons on the double bond are connected to different groups, things get a bit more interesting. The hydrogen atom from the sulfuric acid doesn’t just attach to any carbon; it attaches to the carbon with more hydrogen atoms already attached. This is called Markovnikov’s rule, and it’s a fundamental principle in understanding the reactions of unsymmetrical alkenes.
So, how does this happen? The hydrogen atom from the sulfuric acid adds to the carbon on the double bond that already has more hydrogens. The hydroxyl group (OH) from the sulfuric acid adds to the other carbon. This results in the formation of an alcohol, and the sulfuric acid is regenerated, ready to catalyze another reaction.
It’s important to remember that the structure of the product directly relates to the way you write the formula. Understanding the relationships between structure and formula is a key concept in organic chemistry. It’s a bit like putting together a puzzle; each piece of the structure corresponds to a specific part of the formula.
See more new information: musicbykatie.com
Reaction Of Alkene With Dilute H2So4 | How Does H2So4 React With Alkenes?
Alkene + Dilute H2SO4: A Hydration Reaction
Alkenes are hydrocarbons with a carbon-carbon double bond. They’re pretty reactive, and one of the reactions they undergo is hydration. This is where we add water (H2O) to the alkene.
Dilute H2SO4 is a great way to do this! Sulfuric acid acts as a catalyst, speeding up the reaction.
The Mechanism
Here’s how it works:
1. Protonation: The first step is the protonation of the alkene. A proton (H+) from H2SO4 adds to one of the carbon atoms in the double bond. This forms a carbocation.
2. Nucleophilic Attack: Next, a water molecule acts as a nucleophile and attacks the carbocation. This creates a new carbon-oxygen bond.
3. Deprotonation: Finally, the oxygen atom in the newly formed alcohol is protonated, and a proton is lost, giving us our final product: an alcohol.
Markovnikov’s Rule
Now, you might be wondering: which carbon atom does the proton attach to in the first step? This is where Markovnikov’s rule comes in handy. It states that the proton will attach to the carbon atom that already has the most hydrogen atoms attached to it.
This leads to the formation of the more substituted alcohol. Basically, the alcohol formed will have the hydroxyl group (-OH) attached to the carbon atom with the most alkyl groups.
Examples
Let’s look at some examples to make things clearer:
Ethene (CH2=CH2) reacts with dilute H2SO4 to form ethanol (CH3CH2OH).
Propene (CH3CH=CH2) reacts with dilute H2SO4 to form 2-propanol (CH3CH(OH)CH3).
But-1-ene (CH3CH2CH=CH2) reacts with dilute H2SO4 to form 2-butanol (CH3CH2CH(OH)CH3).
Things to Note
Stereochemistry: The hydration reaction can lead to the formation of both cis and trans isomers of the alcohol.
Reaction Conditions: The reaction is usually carried out at moderate temperatures and under acidic conditions.
Applications:Alkene hydration is an important reaction in the synthesis of alcohols, which are used in many industrial applications.
FAQs
What are the products formed when alkenes react with dilute H2SO4?
The products are alcohols. The position of the hydroxyl group (-OH) is determined by Markovnikov’s rule.
What is the role of H2SO4 in the reaction?
H2SO4 acts as a catalyst, speeding up the reaction, and it also provides the proton needed for the initial protonation of the alkene.
What is the mechanism of the reaction?
The reaction proceeds through a three-step mechanism involving protonation, nucleophilic attack, and deprotonation.
Can dilute H2SO4 be used to hydrate all alkenes?
No, dilute H2SO4 is only effective for hydratingalkenes with terminal double bonds. Internal alkenes (double bonds not at the end of the chain) may require different conditions or catalysts.
What are some industrial applications of alkene hydration?
Alkene hydration is used in the production of alcohols which are used in various industries, including chemicals, pharmaceuticals, and cosmetics.
Can dilute H2SO4 be used to hydratealkynes?
No, dilute H2SO4 is not effective in hydratingalkynes. Alkynes have a triple bond, which requires more force to break.
Summary
So, there you have it! The reaction of alkene with dilute H2SO4 is a hydration reaction that forms alcohols. It’s a useful reaction with several applications in organic chemistry.
Reactions of Alkenes with Sulfuric Acid – Chemistry
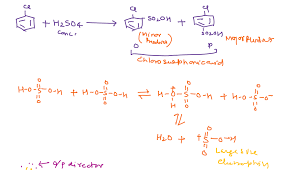
Alkenes react with concentrated sulfuric acid in the cold to produce alkyl hydrogensulphates. For example, ethene reacts to give ethyl hydrogensulphate. \[ \ce{CH_2=CH_2 + H_2SO_4 \rightarrow CH_3CH_2OSO_2OH}\] Chemistry LibreTexts
alkenes and sulphuric (sulfuric) acid – chemguide
Alkenes react with concentrated sulphuric acid in the cold to produce alkyl hydrogensulphates. Ethene reacts to give ethyl hydrogensulphate. The structure of the chemguide
Addition of Sulfuric acid to Alkenes – Chemistry LibreTexts
The reaction with ethene. Alkenes react with concentrated sulfuric acid in the cold to produce alkyl hydrogensulfates. Ethene reacts to give ethyl hydrogensulfate. Chemistry LibreTexts
16.3: Summary of Alkene Reactions – Chemistry
Alkenes are primarily prepared by elimination reactions of molecules that contain good leaving groups attached to sp 3 carbons. Examples of such reactions are dehydrohalogenations with strong base, and acid Chemistry LibreTexts
Reactions of Alkenes – Rutgers University
The reaction of dilute acid to hydrate alkenes is not a fantastic practical route due to insolubility problems, and typically two other indirect approaches are used. (1) Addition crab.rutgers.edu ARCHIVE
Ch15: Hydration of alkenes – Faculty of Science
Reaction type: Electrophilic Addition. Summary. When treated with aq. acid, most commonly H 2 SO 4, alkenes form alcohols. Regioselectivity predicted by Markovnikov’s ucalgary.ca
Hydration (video) | Alkene reactions | Khan Academy
So the way to control your equilibrium is if you want to go to the right, you just dilute your sulfuric acid. You add more water to it, which would increase this concentration. If you Khan Academy
Reaction between Sulphuric Acid and Alkenes – ChemiPhys
The sulphuric acid reacts with the alkene to produce a alkyl hydrogensulphate. This is responsible for the increase in the viscosity of the mixture and the colour change. Two chemiphys.com
4.3.3 Addition Reactions of Alkenes – Save My Exams
The reaction between an alkene and hydrogen is known as hydrogenation or reduction. As well as a nickel catalyst, this requires a temperature of 200 °C and a pressure of 1000 savemyexams.com
Alkene + H2So4 + H2O
A Level Chemistry Revision \”Reaction Between Alkenes And Sulfuric Acid\”
Dil/Conc H²So4 With Alkene || Reaction Of Alcohol With Conc H2So4 || H2So4 Reagents And Application
Potassium Metal Reacts With Concentrated Sulfuric Acid
Alkene + Kmno4 Reaction
Alcohol Dehydration Reaction Mechanism With H2So4
Alkenes: Electrophilic Addition With H2So4 (A-Level Chemistry)
👉Reaction Mechanism(L-9)💚Reaction Of Alkene \U0026 Alkyne With Conc. H2️⃣So4️⃣ \U0026 Dilute H2So4 Or H2O/H+ 💚
Link to this article: reaction of alkene with dilute h2so4.
See more articles in the same category here: https://musicbykatie.com/wiki-how/
