Table of Contents
What is the notation for manganese?
Let’s break down the notation Mn. It’s a chemical symbol, a shorthand way of representing manganese. You’ll see Mn used in chemical formulas, periodic tables, and scientific papers. It’s a universal symbol recognized by scientists worldwide.
You might be curious about why manganese is considered a transition metal. It’s all about its electrons! Transition metals have their outermost electrons in the d orbital, which allows them to form a variety of compounds. These compounds have many important uses in various industries. Think of the vibrant colors in paints, the strength of steel alloys, and even the production of batteries – all these areas utilize manganese in some way!
What is magnesium noble gas notation?
Here’s a breakdown of what that means:
[Ne] represents the electron configuration of the noble gas neon. Neon has 10 electrons, and they are arranged in the following way: 1s², 2s², 2p⁶. This is a very stable arrangement.3s² tells us that magnesium has two additional electrons in the 3s orbital. These are the valence electrons, the ones involved in chemical bonding.
So, magnesium’s full electron configuration is 1s², 2s², 2p⁶, 3s². The noble gas notation is a shortcut, allowing us to focus on the valence electrons.
Let’s delve a bit deeper into how this notation is useful:
Predicting Reactivity: The noble gas notation helps us understand how an element will behave in chemical reactions. Magnesium has two valence electrons, so it tends to lose those electrons to achieve a stable, filled outer shell like neon. This makes magnesium a reactive metal.
Comparing Elements: By comparing the noble gas notation of different elements, we can see how their electron configurations differ and therefore how their chemical properties might vary.
This compact and informative way of representing electron configurations is a valuable tool for chemists and anyone interested in understanding the behavior of elements.
What is the noble gas notation for PB?
We can write the electron configuration for lead as (Xe) 6s2 4f14 5d10 6p2. Let’s break down this configuration:
(Xe): This represents the electron configuration of Xenon, which is a stable noble gas.
6s2: This indicates that there are two electrons in the 6s orbital.
4f14: This signifies that there are 14 electrons in the 4f orbital.
5d10: This tells us that there are 10 electrons in the 5d orbital.
6p2: This indicates that there are two electrons in the 6p orbital.
The noble gas notation for lead is a simplified way to represent its electron configuration by using the previous noble gas and then adding the remaining electrons. This makes it easier to understand the arrangement of electrons in the element, particularly for elements with higher atomic numbers.
How do you write noble gas notation?
For example, let’s take sodium. Its full electron configuration is 1s²2s²2p⁶3s¹. Instead of writing the whole thing out, we can replace the 1s²2s²2p⁶ part with [Ne] because that’s the configuration of neon, the noble gas before sodium. So, sodium’s noble gas configuration becomes [Ne]3s¹.
Essentially, we’re just using the noble gas configuration as a shortcut to represent the core electrons, those closer to the nucleus. This makes it easier to understand the valence electrons, which are the ones involved in chemical bonding.
Let’s break down this concept further.
When we look at the periodic table, noble gases are in group 18. They are special because they have a full outer shell of electrons, making them very stable and unreactive. Remember, atoms want to be stable, so they try to achieve the same electron configuration as the nearest noble gas.
Now, let’s see how we write the noble gas configuration for other elements:
Magnesium (Mg): Full configuration is 1s²2s²2p⁶3s². The noble gas before magnesium is neon (Ne), so we write [Ne]3s².
Chlorine (Cl): Full configuration is 1s²2s²2p⁶3s²3p⁵. The noble gas before chlorine is neon (Ne), so we write [Ne]3s²3p⁵.
The noble gas configuration makes it much easier to visualize the valence electrons. For instance, sodium has one valence electron in the 3s orbital, making it highly reactive as it readily loses this electron to form a cation with a +1 charge.
It’s that easy! By using noble gas notation, you can quickly and easily represent the electron configuration of any atom.
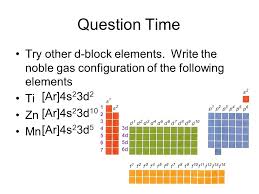
What is manganese noble gas shorthand?
The noble gas shorthand uses the previous noble gas on the periodic table as a starting point. For manganese, the preceding noble gas is argon (Ar). Argon’s electron configuration is 1s22s22p63s23p6. This means we can represent the electron configuration of manganese as [Ar]4s23d5.
This noble gas shorthand makes it easier to visualize the arrangement of electrons in an atom. It also helps us understand the chemical properties of the element. For instance, manganese has one unpaired electron in each of its 3d orbitals, which makes it a transition metal and accounts for its magnetic properties.
Let’s break down why we use noble gases for shorthand. Noble gases are in Group 18 of the periodic table and are known for their stability. They have a full outer shell of electrons, making them unreactive. By using the previous noble gas as a starting point, we essentially ‘skip’ over the filled shells and focus on the electrons that determine an element’s reactivity.
Imagine trying to describe the outfit of a person who is wearing a full suit. Instead of listing every single detail, we could simply say “They’re wearing a suit.” The suit represents the full electron configuration of the previous noble gas, while the details of their shirt and tie represent the additional electrons beyond the filled shell.
This shorthand helps us understand how many electrons are involved in chemical bonding and how these electrons are arranged. It is a valuable tool for chemists and anyone wanting to study the world of atoms.
Which noble gas is MG?
Neon is a noble gas, but magnesium is not. Magnesium is an alkaline earth metal found in Group 2 of the periodic table. Magnesium has two valence electrons, which it readily loses to form a +2 cation, making it highly reactive.
While magnesium does not become a noble gas, it does achieve a stable electronic configuration similar to that of Neon. This means that magnesium, when it loses its two valence electrons, has the same electron configuration as Neon. This is why we say that magnesium achieves a “stable electronic configuration” like a noble gas.
Here’s how magnesium achieves this stability:
Magnesium’s Atomic Structure: Magnesium has 12 electrons arranged in shells: 2 in the first shell, 8 in the second shell, and 2 in the outermost shell (valence shell).
Neon’s Atomic Structure: Neon has 10 electrons: 2 in the first shell and 8 in the second shell.
Magnesium’s Ionization: When magnesium loses its two valence electrons, it becomes a positively charged ion (Mg2+). This leaves magnesium with 10 electrons, identical to the electron configuration of neon.
This similarity in electron configurations explains why magnesium and neon are both very stable. However, magnesium remains an alkaline earth metal, while neon remains a noble gas.
What is the noble gas notation for Mg2+?
Let’s break down why this is significant. Noble gases are a special group of elements found in Group 18 of the periodic table. They are known for their unreactive nature, which is due to their filled outer electron shells. Having a full outer shell makes these elements very stable.
Magnesium, on the other hand, is a reactive metal. It has two electrons in its outer shell. When magnesium loses these two electrons, it forms a cation, Mg²⁺. Losing these electrons allows magnesium to achieve the same stable electronic configuration as Neon. This is why the noble gas notation for Mg²⁺ is [Ne]. This notation is a shorthand way of representing the electronic configuration, indicating that Mg²⁺ has the same electron arrangement as Neon.
Understanding noble gas notation is crucial for comprehending the reactivity and chemical behavior of elements. It helps us understand why certain elements react in specific ways and how they achieve stability.
What is the notation for magnesium?
Magnesium is a chemical element with the symbol Mg and atomic number 12. This means that each magnesium atom has 12 protons in its nucleus. The symbol Mg is a shorthand way to represent magnesium in chemical formulas and equations. You’ll often see it used in chemistry textbooks and lab reports.
Think of it like this: if you were talking about a friend named Bob, you could just use the letter “B” to represent him in a quick text message. Similarly, using “Mg” is a concise way for chemists to represent magnesium.
What is noble gas formula?
The noble gases (previously called inert gases or aerogens) are a group of elements found naturally in Group 18 of the periodic table. They include helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn).
Now, here’s the thing about noble gas formula. These elements are unique because they’re incredibly stable. They have a full outer shell of electrons, making them super resistant to forming bonds with other elements. Think of them as the introverts of the periodic table, content to just be themselves and not needing to interact much. This stability is what makes them “noble” – they don’t need to bond with other elements to become happy and complete.
Because of their lack of reactivity, noble gases don’t typically form compounds with other elements. This is why they are often referred to as inert gases. However, scientists have managed to create some compounds containing xenon and radon under specific conditions. These compounds are quite rare and often exist only in laboratories.
So, while there isn’t a typical “formula” for noble gases like there is for other elements that form compounds, their stability is a key characteristic that sets them apart. Remember, they’re happy being on their own, making them the ultimate chill elements of the periodic table!
See more here: What Is Magnesium Noble Gas Notation? | Noble Gas Notation For Manganese
How to write a notation for manganese atom?
We’ll use the Periodic Table to figure out how many electronsmanganese has. Manganese has 25 electrons. We’ll place these 25 electrons into orbitals around the nucleus of the manganese atom.
Here’s how it works:
1. Start with the first row (period) of the Periodic Table. This row contains hydrogen and helium, and the first orbital is called the 1s orbital. The 1s orbital can hold up to two electrons, so we fill it with two electrons.
2. Move to the second row. This row starts with lithium and ends with neon. The second row has two new orbitals, the 2s orbital and the 2p orbital. The 2s orbital holds two electrons, and the 2p orbital holds six electrons.
3. Continue across the Periodic Table filling each orbital with electrons until you reach manganese.
4. When writing the electron configuration, you list each orbital with the number of electrons it contains. For example, the electron configuration for manganese is 1s²2s²2p⁶3s²3p⁶4s²3d⁵.
Important Note:
When filling orbitals, you follow the Aufbau principle, which states that electrons fill orbitals in order of increasing energy. So, the 1s orbital is filled first, then the 2s orbital, then the 2p orbital, and so on.
You also need to follow Hund’s rule, which states that electrons will occupy each orbital individually before they start to pair up.
Let me know if you need more help writing electron configurations!
What is the electron configuration of manganese?
Manganese (Mn) is a transition metal found in group 7 of the periodic table. It has an atomic number of 25, meaning it has 25 protons and 25 electrons. The electron configuration describes how these electrons are arranged in different energy levels and orbitals around the atom’s nucleus.
The electron configuration of manganese is [Ar] 3d⁵ 4s². This means that:
[Ar] represents the electron configuration of the previous noble gas, argon, which has 18 electrons. So, we’re essentially saying that the first 18 electrons in manganese are arranged the same way as argon.3d⁵ indicates that there are 5 electrons in the 3d orbital, which is the third energy level and has five d orbitals.
4s² indicates that there are 2 electrons in the 4s orbital, which is the fourth energy level and has one s orbital.
Let’s visualize this a bit:
* Imagine the nucleus of manganese at the center of the atom, and the electrons orbiting around it in different energy levels, like shells.
* The first 18 electrons are in the same configuration as argon.
* Then, you have five electrons in the 3d orbital, and two electrons in the 4s orbital.
Now, why is it written as [Ar] 3d⁵ 4s² instead of just 1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁵ 4s²?
This is because writing out the entire electron configuration gets a bit lengthy, especially for heavier elements. Using the noble gas configuration (like [Ar]) is a convenient way to represent the electron configuration by referencing the previous noble gas and only writing the additional electrons. This makes it much easier to understand and compare electron configurations across different elements.
Let me know if you have any more questions about the electron configuration of manganese!
How do you write a configuration for manganese ions?
First, we need to know the electron configuration for a neutral manganese (Mn) atom. We can find the number of electrons for a Mn atom by looking at its position on the Periodic Table. Manganese is in the 7th column of the transition metals, and it’s in the 4th row. That means it has 25 electrons.
Now, let’s write the electron configuration for a neutral Mn atom. The basic principle of writing the configuration is to follow the order of filling the orbitals. The order is represented by the Aufbau principle and can be remembered using the mnemonic diagram.
*1s2 2s2 2p6 3s2 3p6 4s2 3d5*
The electron configuration shows that the Mn atom has 5 electrons in its 3d subshells, and these 5 electrons are all unpaired.
So, when Mn forms an ion, it can lose electrons. The most common manganese ions are Mn2+ and Mn7+.
Mn2+ is formed when Mn loses two electrons. These two electrons come from the 4s orbital. The electron configuration for Mn2+ is:
*1s2 2s2 2p6 3s2 3p6 3d5*
Mn7+ is formed when Mn loses seven electrons. These seven electrons come from the 4s orbital and the 3d orbital. The electron configuration for Mn7+ is:
*1s2 2s2 2p6 3s2 3p6*
The electron configuration of an ion can be used to explain its chemical properties, such as its reactivity and bonding behavior. For example, the electron configuration of Mn2+ shows that it has a stable d5 configuration, which makes it a common oxidation state for manganese.
Let’s dive a little deeper into what we’ve learned about manganese ions:
– Mn2+ is a common ion found in many compounds. It has a stable d5 configuration, meaning it has five unpaired electrons in its d orbital. This configuration is relatively stable, contributing to its presence in various chemical compounds.
– Mn7+ is a highly oxidized state of manganese. It is less common than Mn2+ but still plays a role in certain reactions. Its electron configuration, 1s2 2s2 2p6 3s2 3p6, indicates that it has completely lost all electrons from its d orbitals.
Remember, the electron configuration of an element or an ion can provide valuable information about its chemical properties. Understanding how to write electron configurations and relate them to the periodic table can be a powerful tool for anyone working with chemistry or related fields.
What is the chemical symbol for manganese?
You might be wondering why manganese has that symbol. Well, it comes from the Latin word *magnes*, which means “magnet.” This is because manganese is a ferromagnetic metal, meaning it’s attracted to magnets.
Here’s a bit more about manganese:
Electron configuration: [Ar] 3d5 4s2
Possible oxidation states: +2, +3, +4, +7
This means manganese can lose different numbers of electrons to form ions. The most common oxidation state is +2, but it can also exist in higher oxidation states, which is important in its chemical reactions.
Let’s talk about how the electron configuration of manganese helps us understand its behavior:
[Ar]: This part tells us that manganese has the same electron configuration as argon, which is a noble gas. Noble gases are very stable and unreactive because they have a full outer shell of electrons.3d5 4s2: This part describes the arrangement of the electrons in manganese’s outer shells. The 3d subshell has 5 electrons and the 4s subshell has 2 electrons. This arrangement gives manganese a unique ability to form a variety of compounds and participate in various chemical reactions.
It’s important to know that manganese is a transition metal, which means it has a partially filled d subshell. This makes it a key ingredient in many alloys, pigments, and even vitamins. It’s also essential for plant growth and plays a vital role in various biological processes within our bodies.
See more new information: musicbykatie.com
Noble Gas Notation For Manganese: A Step-By-Step Explanation
You see, noble gas notation is a super handy way to represent the electron configuration of an element. It’s a bit like using a shortcut to write down the arrangement of electrons in an atom. So, what’s the deal with manganese? Well, it’s a transition metal, which means it’s got some interesting properties.
Let’s break it down:
1. Finding the Noble Gas: The first step is to locate the nearest noble gas on the periodic table that comes before manganese. You know, those elements in group 18, the ones that are super happy with their electron configuration? For manganese (Mn), that noble gas is argon (Ar).
2. Writing the Noble Gas Symbol: Now we write the symbol of that noble gas in square brackets, like this: [Ar].
3. Identifying the Remaining Electrons: The next thing we do is figure out how many electrons are left in manganese after we’ve accounted for those in argon. Manganese has 25 electrons. Argon has 18 electrons. So, we have 25 – 18 = 7 electrons left to deal with.
4. Filling the Orbitals: Now we have to fill the remaining orbitals with those 7 electrons. Manganese has the electron configuration of [Ar] 3d⁵ 4s², meaning it has 5 electrons in the 3d subshells and 2 electrons in the 4s subshells.
So, there you have it! The noble gas notation for manganese is [Ar] 3d⁵ 4s². It’s a pretty efficient way to describe the electron configuration, right?
Let’s take a look at why noble gas notation is so useful. It makes things a lot easier:
* It’s concise: You don’t need to write out the entire electron configuration every time. Just the noble gas symbol and the remaining electrons do the trick.
* It’s clear: You can easily see how many electrons are in each subshell.
* It helps you understand trends: You can quickly see how the electron configuration of an element relates to its position on the periodic table.
A Deeper Dive
Let’s talk a bit more about the electron configuration of manganese. It’s actually a bit tricky because it doesn’t follow the typical “Aufbau principle.” You know, the principle that says electrons fill orbitals in order of increasing energy.
Manganese’s electron configuration seems a bit strange at first glance. Why is it [Ar] 3d⁵ 4s² instead of [Ar] 3d⁴ 4s²? The reason lies in something called Hund’s rule, which states that electrons prefer to occupy separate orbitals within a subshell, rather than pairing up in the same orbital.
Think of it like this: Imagine you have three seats on a bus. It’s more comfortable for three people to sit in their separate seats rather than two people cramming into one seat and the other person having to stand.
Manganese has five electrons in the 3d subshell. These five electrons prefer to occupy separate 3d orbitals, rather than pairing up in the same orbital. This leads to a more stable configuration, which is why manganese’s electron configuration is [Ar] 3d⁵ 4s².
What about other transition metals?
The same principles apply to other transition metals. For example, the noble gas notation for iron (Fe) is [Ar] 3d⁶ 4s².
Remember, noble gas notation is a really helpful tool for understanding the electron configuration of elements, especially for those tricky transition metals!
FAQs
Q: What is the difference between electron configuration and noble gas notation?
A: Electron configuration describes the arrangement of electrons in an atom, listing all occupied subshells and their corresponding numbers of electrons. Noble gas notation uses the noble gas symbol for the previous period to simplify this notation.
Q: Why is noble gas notation called “noble gas notation?”
A: Because it uses the symbols of noble gases, which are the most stable elements in the periodic table due to their full outer shells of electrons.
Q: What is the electron configuration of manganese?
A: The electron configuration of manganese is 1s²2s²2p⁶3s²3p⁶4s²3d⁵ or [Ar] 3d⁵ 4s² using noble gas notation.
Q: What is the electron configuration of iron?
A: The electron configuration of iron is 1s²2s²2p⁶3s²3p⁶4s²3d⁶ or [Ar] 3d⁶ 4s² using noble gas notation.
Q: Why is Hund’s rule important?
A: Hund’s rule is important because it helps us understand the electron configuration of elements, especially transition metals, which have multiple electrons in the d orbitals. It explains why electrons prefer to occupy separate orbitals within a subshell, rather than pairing up in the same orbital, leading to a more stable configuration.
Q: Can you give me an example of how noble gas notation is used in chemistry?
A: Certainly! One way noble gas notation is used in chemistry is to predict the reactivity of elements. Elements with a full outer shell, such as noble gases, are generally unreactive. This is because they have a stable electron configuration and don’t need to gain or lose electrons to become more stable. Elements with incomplete outer shells, on the other hand, are more reactive. They may gain or lose electrons to achieve a full outer shell and become more stable. So, if we know the electron configuration of an element, we can predict how it might react with other elements.
Q: How can I learn more about noble gas notation?
A: There are many resources available online and in textbooks that can help you learn more about noble gas notation. You can also check out educational videos on YouTube or Khan Academy.
I hope this explanation was helpful! Now you should be able to confidently explain the noble gas notation for manganese and understand its significance in chemistry.
How do you write the noble-gas electron configuration for
Explanation: …the nearest Noble gas is argon, Z=18 .. And so we do not have to specify the configuration of the first 18 electrons, because these approximate the configuration of argon.. Z = 18,1s22s22p63s23s6 electronic configuration of argon. For Socratic
Electron Configuration Chart of All Elements (Full Chart)
The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also mentioned in the table. Periodic Table Guide
5.20: Noble Gas Configuration – Chemistry LibreTexts
This provides the basis for a shorthand notation for electron configurations called the noble gas configuration. The elements that are found in the last column of the periodic table are an important group of elements called Chemistry LibreTexts
Electron Configuration for Mn, Mn2+, Mn3+ , and
To write the configuration for the Manganese ions, first we need to write the electron configuration for just Manganese (Mn). We first need to find the number of electrons for the Mn atom… YouTube
Electron Configuration for Manganese (Mn, Mn2+,
The electron configuration of manganese shows that the last shell of manganese has two electrons and the d-orbital has a total of five electrons. Therefore, the valence electrons of manganese are seven. Valenceelectrons.com
What is the Noble Gas notation (Electron configuration) for the …
Well what is #Z# for manganese….? Explanation: My Periodic Table tells me #Z=25# …and if there are 25 positive, nuclear charges, there are 25 electronic charges, Socratic
Manganese – Electron Configuration and Oxidation States – Mn
For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used. The electron configuration can be visualized as the core electrons, Periodic Table of Elements
Manganese – Element information, properties and uses | Periodic
The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. Boiling point The The Royal Society of Chemistry
Noble gas – Wikipedia
The noble gases (historically the inert gases, sometimes referred to as aerogens [1]) are the naturally occurring members of group 18 of the periodic table: helium (He), neon Wikipedia
Electron Configuration With Noble Gas Notation
Noble Gas Configuration | Electronic Structure Of Atoms | Chemistry | Khan Academy
Noble Gas Notation
Electron Configuration For Mn, Mn2+, Mn3+ , And Mn4+ (Manganese And Manganese Ions)
Write A Noble Gas Or Shorthand Electron Configuration Arsenic
Pseudo Noble Gas Electron Configurations
Manganese Ground State Electron Configuration
Noble Gas Notation – Ap Chemistry
Link to this article: noble gas notation for manganese.
See more articles in the same category here: https://musicbykatie.com/wiki-how/
