Table of Contents
How is NO2 mixed anhydride?
NO2, or nitrogen dioxide, is a fascinating molecule. When it dissolves in water, it doesn’t just sit there, it actually gets busy and forms a mix of HNO2 (nitrous acid) and HNO3 (nitric acid). This is why we can consider it a mixed anhydride – it’s like a chemical chameleon, capable of morphing into two different acids.
But what exactly *is* a mixed anhydride? Think of it like a bridge between two different things. In this case, the bridge is NO2, and it connects HNO2 and HNO3.
An anhydride is a compound that can form an acid when you add water. A mixed anhydride has the ability to form *two* different acids when it reacts with water. NO2, in this way, acts as a link between HNO2 and HNO3. It’s like a molecular connector, letting these two acids share a common starting point.
This behavior of NO2 is a bit like a chemical magic trick. It starts off as one thing (NO2) and then transforms into two different things (HNO2 and HNO3) when it meets water. This ability to form a mixture of acids makes NO2 a mixed anhydride, showcasing its unique chemical personality.
Why is N2O4 mixed anhydride?
Nitrogen dioxide (NO2), when reacted with water, forms a mixture of two acids: nitric acid (HNO3) and nitrous acid (HNO2). This is because NO2 can act as both an oxidizing agent and a reducing agent, leading to the formation of these two acids. This makes N2O4 (which is in equilibrium with 2NO2) a mixed anhydride, as it produces a mixture of acids upon reaction with water.
Here’s a more detailed explanation:
Anhydrides are compounds that react with water to form acids. They are essentially oxides of non-metals.
Mixed anhydrides are anhydrides that form a mixture of two or more acids when reacting with water.
The reaction of N2O4 with water can be represented by the following equation:
N2O4 + H2O → HNO3 + HNO2
In this reaction, N2O4 acts as a mixed anhydride because it forms both nitric acid (HNO3) and nitrous acid (HNO2).
Here’s how it works:
NO2 can act as an oxidizing agent, leading to the formation of HNO3. In this process, NO2 is reduced to NO, which further reacts with water to form HNO2.
NO2 can also act as a reducing agent, leading to the formation of HNO2. In this process, NO2 is oxidized to NO3-, which combines with a proton (H+) to form HNO3.
The formation of both nitric acid and nitrous acid through these reactions explains why N2O4 is classified as a mixed anhydride. It’s important to note that the reaction of N2O4 with water is an equilibrium reaction, meaning that the products can react to form the reactants again.
Therefore, the mixture will contain a combination of HNO3, HNO2, and N2O4 in equilibrium.
Why is NO2 called double acid anhydride?
You’re right, NO2 is called a double acid anhydride because when it reacts with water, it forms two acids: nitrous acid (HNO2) and nitric acid (HNO3). This is a neat chemical reaction!
Let’s break down why this happens. When NO2 reacts with water, it undergoes a disproportionation reaction. This means that the nitrogen atom in NO2 is both oxidized and reduced in the same reaction. Here’s a simplified explanation of what happens:
One molecule of NO2 gets reduced to form nitrous acid (HNO2), where the nitrogen atom has a lower oxidation state (+3).
Another molecule of NO2 gets oxidized to form nitric acid (HNO3), where the nitrogen atom has a higher oxidation state (+5).
This reaction is important because it showcases the versatility of NO2. This molecule can act as both an oxidizing agent and a reducing agent, depending on the reaction conditions.
The formation of two acids from a single molecule of NO2 is what earns it the name double acid anhydride.
What is the name of the anhydride of HNO2?
N2O3, also known as dinitrogen trioxide, is the anhydride of HNO2 (nitrous acid). This means that when N2O3 reacts with water, it forms HNO2. The reaction can be represented by the following chemical equation:
N2O3 + H2O → 2 HNO2
Anhydrides are essentially acidic oxides. They are formed when water is removed from an acid. In the case of HNO2, removing water leads to the formation of N2O3.
N2O3 is a blue gas at room temperature. It is unstable and readily decomposes into NO (nitric oxide) and NO2 (nitrogen dioxide). This decomposition is particularly favored at higher temperatures. N2O3 also exists in liquid form, which is a dark blue liquid.
Let’s explore the relationship between anhydrides and their corresponding acids in more detail.
Anhydrides are formed when water is removed from two molecules of an acid. This process is called dehydration. For example, when two molecules of HNO2 lose a water molecule, they form N2O3.
Conversely, when an anhydride reacts with water, it forms the corresponding acid. This process is called hydration. For example, when N2O3 reacts with water, it forms HNO2.
Here’s a simple analogy to help you understand the concept:
Imagine you have a cup of coffee. The coffee itself is the acid. Now, imagine that you remove the water from the coffee. What you’re left with is the anhydride, which is essentially the concentrated coffee essence. When you add water back to the coffee essence, you get your original coffee back. This is similar to the relationship between anhydrides and their corresponding acids.
What is the mixed anhydride method?
1. Activation of the carboxyl component: This involves transforming the carboxyl group of an Nα-protected amino acid or peptide into a reactive derivative. This step is crucial because the carboxyl group, in its original form, isn’t reactive enough to directly form a peptide bond.
2. Generation of a mixed anhydride: This is where the “mixed” part of the name comes in. The activated carboxyl group reacts with an anhydride, resulting in the formation of a mixed anhydride. This mixed anhydride is a highly reactive species that’s ready to react with the amine component.
3. Coupling with the amine component: The final step involves reacting the mixed anhydride with the amine component, which is typically another Nα-protected amino acid or peptide. This reaction forms the peptide bond, linking the two components together.
The mixed anhydride method is a versatile technique that can be used to synthesize a wide range of peptides. It’s particularly useful for creating peptides with complex sequences or those containing sensitive functional groups. One of the key advantages of this method is that it produces minimal racemization, which is a side reaction that can lead to the formation of unwanted isomers.
Let’s delve a bit deeper into the steps:
Activation of the carboxyl component: A common method for activating the carboxyl group involves using a reagent like dicyclohexylcarbodiimide (DCC). DCC reacts with the carboxyl group to form an O-acylisourea, a highly reactive intermediate.
Generation of a mixed anhydride: The O-acylisourea then reacts with a carboxylic acid, such as acetic acid or pivalic acid, to form a mixed anhydride. This mixed anhydride is highly reactive and ready for the next step.
Coupling with the amine component: The mixed anhydride reacts with the amine component, which is typically another Nα-protected amino acid or peptide. This reaction forms a peptide bond, linking the two components together.
The mixed anhydride method is a popular choice for peptide synthesis due to its efficiency and effectiveness. It’s a reliable way to form peptide bonds with minimal side reactions, making it a valuable tool for researchers and scientists working in various fields, such as drug discovery, biotechnology, and materials science.
How to identify a mixed anhydride?
A mixed anhydride is a compound formed when two different carboxylic acids combine, losing a water molecule in the process. For example, the reaction of acetic acid and propionic acid will yield a mixed anhydride, acetic propionic anhydride.
How do you spot a mixed anhydride? The key is to look for the presence of two different acyl groups attached to the same oxygen atom. An acyl group is essentially a carbonyl group (C=O) with an alkyl group (R) attached to it.
Imagine a molecule with a central oxygen atom. On one side, you’ll find an acyl group from one carboxylic acid. On the other side, you’ll see a different acyl group from a second carboxylic acid. That’s your mixed anhydride!
For instance, let’s consider acetic propionic anhydride. One side has the acetyl group (CH3CO-), derived from acetic acid. The other side has the propionyl group (CH3CH2CO-), originating from propionic acid. This unique combination tells us we’re dealing with a mixed anhydride.
What is the difference between anhydride and mixed anhydride?
Think of it like this: imagine you have two different types of cookies, chocolate chip and peanut butter. If you combine them together, you get a mixed cookie. It’s still a cookie, but it’s a combination of two different types. The same principle applies to anhydrides. A mixed anhydride is essentially a combination of two different carboxylic acid anhydrides.
Let’s dive a little deeper into the concept of mixed anhydrides.
You can visualize a mixed anhydride as a bridge between two different carboxylic acids. One end of the bridge is linked to one acid, and the other end is linked to the second acid. The oxygen atom in the middle acts as the bridge, connecting the two ends.
Mixed anhydrides are particularly useful in organic chemistry because they can be used to create a variety of different compounds. Here’s how it works:
1. Reactivity:Mixed anhydrides are more reactive than regular anhydrides because the two acyl groups can react with each other.
2. Selectivity: The different acyl groups in a mixed anhydride allow for a degree of control in which group reacts first. This is helpful in synthesizing complex molecules.
3. Diversity: By varying the two carboxylic acids used to make the mixed anhydride, you can create a wide range of different compounds. This makes mixed anhydrides a versatile tool in organic synthesis.
So, in a nutshell, a mixed anhydride is simply an anhydride that’s formed from two different carboxylic acids. It’s a valuable tool in organic chemistry because it offers greater reactivity and selectivity compared to regular anhydrides.
What is an example of a mixed anhydride?
Imagine it like a bridge connecting two different things. In this case, the bridge is the oxygen atom, and the two different things are the acid groups. These acid groups can be inorganic or organic, adding to the diversity of mixed anhydrides.
For instance, dinitrogen tetroxide, with the formula N₂O₄, is a mixed anhydride of nitric acid (HNO₃) and nitrous acid (HNO₂). On the other hand, chloryl perchlorate, ClO₂ClO₄, is a mixed anhydride of perchloric acid (HClO₄) and chloric acid (HClO₃).
Mixed anhydrides play a crucial role in various chemical reactions, acting as important reagents and intermediates. Their reactivity stems from the presence of two different acid groups, allowing them to participate in a wide range of transformations. These transformations can include nucleophilic attacks, acylations, and other reactions that are essential in organic and inorganic chemistry.
Acyl groups, as mentioned in the original text, are important in organic chemistry. They are groups of atoms with the structure R-C=O, where R represents any organic group. In acid anhydrides, two acyl groups are connected by an oxygen atom. These compounds are known for their role in reactions that introduce acyl groups, making them key players in organic synthesis.
It’s important to remember that the structure and reactivity of mixed anhydrides can be quite varied. Understanding the nature of the acid groups involved and the overall structure of the compound is crucial for predicting its reactivity and potential applications.
See more here: How Is No2 Mixed Anhydride? | Mixed Anhydride Of Nitrous And Nitric Acid
How does nitrous oxide react with water?
Nitrogen(III) oxide (N₂O₃) is the anhydride of nitrous acid (HNO₂). This means that when N₂O₃ reacts with water, it forms HNO₂.
Nitrogen(IV) oxide (NO₂) is a bit more interesting. Because there are no stable oxyacids containing nitrogen with an oxidation state of +4, NO₂ undergoes a process called disproportionation when it reacts with water. This means that the NO₂ molecules react with each other, with some acting as oxidizing agents and others acting as reducing agents. The result is a mixture of products.
In cold water, the reaction produces a mixture of nitrous acid (HNO₂) and nitric acid (HNO₃).
Here’s a closer look at what’s happening:
1. NO₂ molecules are highly reactive and readily dissolve in water.
2. In the presence of water, some NO₂ molecules get oxidized to nitric acid (HNO₃), while others get reduced to nitrous acid (HNO₂) .
3. The overall reaction can be represented by the following equation:
3 NO₂ + H₂O → 2 HNO₃ + NO
This reaction is an equilibrium reaction. The relative amounts of HNO₃ and HNO₂ produced depend on the temperature and the concentration of the reactants.
Here’s a more detailed explanation of the process:
Oxidation: In this step, NO₂ acts as a reducing agent, meaning it loses electrons and gets oxidized to HNO₃.
Reduction: In this step, NO₂ acts as an oxidizing agent, meaning it gains electrons and gets reduced to HNO₂.
Disproportionation: This is the overall process in which NO₂ molecules react with each other, leading to both oxidation and reduction reactions.
Understanding disproportionation is key to understanding how NO₂ interacts with water. The reaction produces a mixture of acids, which can have different properties and effects. For example, nitric acid is a strong acid, while nitrous acid is a weak acid.
Which two oxides of nitrogen are stable oxyacids?
Two nitrogen oxides are acid anhydrides, meaning they react with water to form nitrogen-containing oxyacids.
Dinitrogen trioxide is the anhydride of nitrous acid (HNO2).
Dinitrogen pentoxide is the anhydride of nitric acid (HNO3).
Here’s how the reaction with dinitrogen pentoxide works:
N2O5 + H2O → 2HNO3
Now, let’s address the missing piece about stable oxyacids. While nitrous and nitric acid are the most common oxyacids of nitrogen, there are other, less stable ones that can exist. These less stable oxyacids tend to be highly reactive and decompose easily.
Here’s why this happens:
Nitrogen’s oxidation state: Nitrogen can exist in various oxidation states, ranging from -3 to +5. The stability of oxyacids depends on the oxidation state of nitrogen. Nitrous acid (HNO2) and nitric acid (HNO3) represent nitrogen in its +3 and +5 oxidation states, respectively. These states are relatively stable, making the acids stable.
Bond strength: The strength of the bonds between nitrogen and oxygen in the oxyacids plays a crucial role. Stronger bonds lead to greater stability. Nitrous acid and nitric acid have relatively strong N-O bonds, contributing to their stability.
Acid strength: The strength of an acid is another factor. Nitrous and nitric acid are moderately strong acids. They are not the strongest acids, but their stability is linked to this moderate strength.
So, while it’s true that nitrous and nitric acid are the most stable oxyacids, there are other oxyacids of nitrogen that exist, even if they are less stable and more prone to decomposition. The stability of these oxyacids is determined by a combination of factors, including nitrogen’s oxidation state, bond strength, and the strength of the acid itself.
How does nitrous acid decompose?
Let’s break down what’s happening in this decomposition:
Nitrous acid (HNO2): It’s a weak acid that doesn’t fully break apart (dissociate) in water. This means that it exists in a state of equilibrium between the undissociated HNO2 and its ions, H+ and NO2-.
Nitric acid (HNO3): This is a stronger acid, formed as a result of the decomposition of HNO2. The formation of HNO3 is favored at higher temperatures.
Nitric oxide (NO): This is a colorless gas formed by the decomposition of HNO2. NO plays a vital role in various chemical reactions and atmospheric processes.
Water (H2O): This is a byproduct of the decomposition reaction.
How the Decomposition Happens
The decomposition of nitrous acid is a complex process that involves several steps. Essentially, the process involves the breaking of the N-O bond in HNO2, resulting in the formation of NO and OH. The OH radical can then react with another HNO2 molecule, leading to the formation of HNO3 and H2O.
Factors Affecting Decomposition
Several factors influence the rate at which nitrous acid decomposes. These include:
Temperature: As we already discussed, increasing the temperature boosts the decomposition rate.
Concentration: Higher concentrations of nitrous acid lead to a faster decomposition rate. The more HNO2 molecules present, the more likely they are to collide and react, leading to decomposition.
pH: The acidity of the solution (pH) also plays a role. A lower pH (more acidic) favors the decomposition of HNO2.
Presence of Catalysts: Certain substances can act as catalysts, speeding up the decomposition rate without being consumed in the process.
Understanding how nitrous acid decomposes is important for various applications, including chemical synthesis, environmental chemistry, and industrial processes. It also helps us understand the role of nitrous acid in atmospheric chemistry, where its decomposition contributes to the formation of ozone and other atmospheric pollutants.
See more new information: musicbykatie.com
Mixed Anhydride Of Nitrous And Nitric Acid | What Is The Acidic Anhydride Of Nitrous And Nitric Acid?
Okay, let’s talk about something pretty interesting – mixed anhydrides! But before we dive into the nitty-gritty of these special compounds, let’s define what they are first.
A mixed anhydride is a chemical compound that’s like a hybrid, formed by combining two different acid anhydrides. An acid anhydride is basically what you get when you remove a water molecule from two acid molecules.
So, a mixed anhydride has this unique structure where you have two different acid groups linked together. It’s like a molecular sandwich, with each slice representing a different acid.
Now, let’s talk about our star player – the mixed anhydride of nitrous and nitric acid. This fascinating compound is a bit of a chemical mystery, and it’s not as common as its simpler counterparts, like acetic anhydride.
You might be wondering why we don’t see it around as much. Well, it turns out that this mixed anhydride is quite unstable. It’s like a volatile cocktail – easily prone to decomposition. Think of it like a delicate balance that’s easily tipped.
The Decomposition Dance:
The breakdown of the mixed anhydride can happen in a couple of ways. It can either decompose into its parent acids – nitrous and nitric acid – or it can react with itself to produce nitrogen dioxide and nitrogen tetroxide. This decomposition can be influenced by factors like temperature and the presence of other compounds.
Where Does It Fit In?
So, you might be thinking, “Okay, this mixed anhydride is a bit of a chemical wildcard. What’s its role in the grand scheme of things?”
Well, while it might not be a household name, it does have a bit of a spotlight moment in the world of chemical reactions. It’s a potential intermediate in various reactions involving nitrous and nitric acid. That means it can pop up briefly during a reaction, acting like a bridge connecting the two parent acids.
A Peek into the Nitrous Acid World:
Now, let’s talk about nitrous acid (HNO₂). It’s a weak acid, which means it doesn’t completely ionize in solution. It’s a bit of a shy player, not as strong as its brother, nitric acid (HNO₃). Nitrous acid is known for its role in reactions like diazotization, where it helps form diazonium salts – important compounds used in various industries.
A Deeper Dive into Nitric Acid:
Nitric acid, on the other hand, is a strong acid, readily donating its proton (H⁺) in solution. It’s a bit of a workhorse in chemical reactions, featuring prominently in the production of fertilizers, explosives, and even some dyes.
The Synthesis Puzzle:
You might be wondering how we even make this mixed anhydride. Well, it’s not a straightforward process, and there are a couple of ways to try. One approach involves reacting nitrous acid with nitric anhydride, which can be a bit tricky due to the inherent instability of these compounds.
Another approach involves using a reaction between nitric acid and a nitrating agent like potassium nitrite (KNO₂). But remember, it’s like trying to catch a fleeting shadow – this mixed anhydride is pretty transient and can quickly disappear.
Unveiling the Mystery:
So, how do we know this elusive mixed anhydride even exists? Well, it’s a bit of a detective story. Researchers have used techniques like spectroscopy, particularly infrared spectroscopy, to detect its presence in different reaction mixtures. It’s like taking a chemical fingerprint – analyzing the unique patterns of light absorption to identify the mixed anhydride.
The End of Our Journey:
So, there you have it! The mixed anhydride of nitrous and nitric acid is a bit of a chemical enigma – a fleeting compound with a short-lived existence. But it’s a fascinating example of the complex and interconnected nature of chemistry, where even seemingly obscure compounds can play a role in the grand scheme of things.
FAQs about Mixed Anhydride of Nitrous and Nitric Acid
1. What are the main uses of the mixed anhydride of nitrous and nitric acid?
While the mixed anhydride itself is not widely used, it’s believed to be a potential intermediate in various chemical reactions involving nitrous and nitric acid, particularly in the synthesis of nitrogen oxides.
2. Is it possible to isolate the mixed anhydride of nitrous and nitric acid?
Due to its instability, isolating this mixed anhydride in a pure form is extremely challenging. It’s often detected as a transient intermediate in reactions and not as a stable isolable compound.
3. What are the safety hazards associated with the mixed anhydride of nitrous and nitric acid?
The mixed anhydride is a highly reactive compound that can decompose explosively. It’s also corrosive and can be harmful if inhaled or ingested. It’s essential to handle this compound with extreme caution and follow proper safety protocols.
4. How can I learn more about the mixed anhydride of nitrous and nitric acid?
You can explore chemical literature, particularly research papers and textbooks that specialize in inorganic chemistry or the chemistry of nitrogen oxides.
5. Is there a specific application for this mixed anhydride in industry?
Currently, there are no specific industrial applications that directly utilize this mixed anhydride. It’s mainly a topic of interest for research and understanding the reactivity of nitrogen oxides.
6. What are some key characteristics of the mixed anhydride of nitrous and nitric acid?
Key characteristics include its instability, reactivity, and transient nature. It’s known to decompose rapidly into its parent acids or other nitrogen oxides.
7. What is the chemical formula of the mixed anhydride of nitrous and nitric acid?
The chemical formula of this mixed anhydride is HNO₂-ONO₂, representing the combination of nitrous and nitric acid moieties.
8. Why is it called a “mixed anhydride?”
The term “mixed anhydride” highlights the fact that this compound is formed by combining two different acid anhydrides, in this case, nitrous acid anhydride and nitric acid anhydride.
9. How is the mixed anhydride of nitrous and nitric acid related to nitrogen oxides?
It’s a potential intermediate in reactions involving nitrous and nitric acid, which can lead to the formation of nitrogen oxides like nitrogen dioxide (NO₂) and nitrogen tetroxide (N₂O₄).
10. What are some of the challenges associated with studying the mixed anhydride of nitrous and nitric acid?
Its instability and short-lived nature make it difficult to study directly. Specialized techniques, like spectroscopy, are necessary to detect its presence in reactions.
Nitrogen trioxide – N2O3, Structure, Molecular Mass, Physical and …
It is produced as an anhydride when the unstable nitrous acid is mixed in water. If the nitrous acid (HNO 2) can decompose into nitric acid and nitric oxide. Nitrite salts BYJU’S
The mixed anhydride of nitrous and nitric acid is – Infinity Learn
The mixed anhydride of nitrous and nitric acid is. Moderate. A. N 2 O. B. NO 2. C. NO. D. N 2 O 5. Solution. HNO 2 + HNO 3 NO 2 + H 2 O. Hence NO 2 is mixed anhydride Infinity Learn
18.7: Occurrence, Preparation, and Properties of Nitrogen
Nitrogen(III) oxide, N 2 O 3, is the anhydride of nitrous acid; HNO 2 forms when N 2 O 3 reacts with water. There are no stable oxyacids containing nitrogen with an oxidation Chemistry LibreTexts
What are mixed anhydrides? – Vedantu: Online Courses
Here, two acids which are formed are known as nitrous acid $(HN{O_2})$ and nitric acid $(HN{O_3})$. Let’s see some more examples for mixed anhydrides: Vedantu
Notes on Dinitrogen Trioxide, N2O3 – Unacademy
When water and unstable nitrous acid are mixed together, it produces anhydride. HNO2 (nitrous acid) can be broken down into nitric acid and nitric oxide. Mixing N2O3 with Unacademy
Mixed Anhydride – Chemistry LibreTexts
A mixed anhydride is a carboxylic acid anhydride that has the following general structural formula. R 1 ≠R 2 =hydrogen atoms, alkyl groups, aryl groups. eg: see also symmetrical anhydride Chemistry LibreTexts
20.18: Reactions of Anhydrides – Chemistry LibreTexts
If you took two ethanoic acid molecules and removed a molecule of water between them you would get the acid anhydride, ethanoic anhydride (old name: acetic anhydride). Chemistry LibreTexts
Acid anhydride | chemical compound | Britannica
In oxide: Oxides of nitrogen. Two oxides of nitrogen are acid anhydrides; that is, they react with water to form nitrogen-containing oxyacids. Dinitrogen trioxide is the anhydride of Britannica
The Mixed Anhydride Of Nitrous And Nitric Acid Is.
`X Rarr` Mixed Anhydride Of Nitrous Acid And Nitric Acid `Y Rarr` Non-Aq Solvent Obtained On
The Mixed Anhydride Of Nitrous And Nitric Acid Is. | 12 | P-Block Group 15 Elements – The Nitrog…
The Mixed Anhydride Of Nitrous And Nitric Acid Is. | 12 | P Block Elements | Chemistry | Resonan…
Which Of The Following Is An Anhydride Of Nitrous Acid? A. N_2O_3 B. No C. No_2 D. N_2O_5
Which Among Th Following Is Mixed Anhydride? | 12 | Nitrogen \U0026 Oxygen Family | Chemistry | Reson…
`No_(2)` Is The Mixed Anhydride Of And Acids.
Which Among The Following Is Mixed Anhydride? | 12 | Nitrogen \U0026 Oxygen Family | Chemistry | Reso…
Link to this article: mixed anhydride of nitrous and nitric acid.
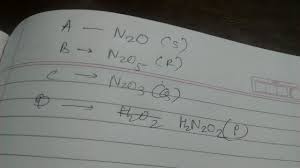
See more articles in the same category here: https://musicbykatie.com/wiki-how/
