Table of Contents
How is nitrobenzene synthesized?
Nitrobenzene is commercially manufactured by the direct nitration of benzene using mixed acid (a blend of nitric acid (HNO3) and sulfuric acid (H2SO4)). This reaction is like a chemical handshake, where the mixed acid delivers a nitro group (NO2) to the benzene molecule, transforming it into nitrobenzene.
Historically, nitrobenzene was made through a batch process. In this method, a reactor is filled with benzene and heated to a temperature between 50-55 °C. Then, the mixed acid is slowly added to the reactor, carefully controlling the rate of reaction to prevent runaway conditions.
The mixed acid acts as both a nitrating agent and a dehydrating agent. The nitric acid provides the nitro group, and the sulfuric acid removes water produced during the reaction, driving the equilibrium towards nitrobenzene formation. The reaction is usually carried out at a temperature between 50-60 °C, and the nitrobenzene product is then separated from the reaction mixture through distillation.
This batch process was effective but had some limitations. It required careful monitoring and control to prevent undesirable side reactions, and the equipment had to be thoroughly cleaned between batches. Modern production methods have largely transitioned to continuous processes, which offer significant advantages in terms of efficiency and control.
In continuous processes, benzene and mixed acid are fed into a reactor continuously, allowing for more precise control over the reaction conditions. The continuous flow also reduces the risk of side reactions and allows for more efficient separation of the nitrobenzene product.
While the process may seem straightforward, the key is in the careful selection and control of the reaction conditions. Factors like temperature, concentration of reactants, and the presence of impurities can all have a significant impact on the yield and purity of the nitrobenzene produced.
Understanding the chemistry and engineering behind nitrobenzene production is essential for its safe and efficient production, which plays a vital role in various industries.
Which of the following reagent can be used to convert nitrobenzene?
Let’s break down how this works. Tin acts as a reducing agent, providing electrons to the nitro group. Hydrochloric acid acts as a proton source, providing hydrogen ions (H+) to complete the reduction process. The reaction can be represented as follows:
“`
C6H5NO2 + 6[H] → C6H5NH2 + 2H2O
“`
Here, the [H] represents the reducing agent, which in this case is provided by tin and hydrochloric acid. The reaction produces aniline (C6H5NH2) and water (H2O) as byproducts.
The reduction of nitrobenzene to aniline is an important reaction in organic chemistry, as it allows us to synthesize aniline, a versatile building block for various organic compounds. Aniline is used in the production of dyes, pharmaceuticals, and polymers.
This reaction is a classic example of a reduction reaction and highlights the important role of tin and hydrochloric acid as reducing agents in organic chemistry. Understanding this reaction provides a foundation for exploring other reduction reactions and their applications in the synthesis of various organic compounds.
Does benzene require a catalyst?
Electrophilic Aromatic Substitution
Benzene can undergo electrophilic aromatic substitution reactions. These are reactions where an electrophile, an electron-seeking species, replaces one of the hydrogen atoms on the benzene ring. The problem is, some electrophiles, like halogens, aren’t strong enough to break the aromaticity of benzene on their own.
The Need for a Catalyst
This is where catalysts come in. A catalyst is a substance that speeds up a chemical reaction without being consumed in the process. In the case of halogenation of benzene, a catalyst is required to activate the halogen and make it reactive enough to attack the benzene ring.
How Catalysts Work
These catalysts work by forming a more reactive species, often by reacting with the halogen to create a more electrophilic intermediate. For instance, when brominating benzene, a common catalyst is iron tribromide (FeBr₃). The iron tribromide reacts with the bromine to form a more electrophilic bromine species, which then attacks the benzene ring. This reaction replaces one of the hydrogen atoms with a bromine atom.
Understanding the Chemistry
Let’s break down the chemistry a bit further. The FeBr₃ reacts with bromine to create a positively charged bromine species (Br⁺) and a complex anion (FeBr₄⁻). This positively charged bromine species is much more electrophilic than elemental bromine and can easily attack the benzene ring.
Key Takeaway
So, while benzene is quite stable, it can still undergo electrophilic aromatic substitution reactions. However, reactions with weak electrophiles, like halogens, require a catalyst to activate the electrophile and facilitate the reaction.
How do you convert benzene to aniline?
First, you need to treat benzene with a mixture of concentrated nitric acid (HNO3) and concentrated sulfuric acid (H2SO4). This process is called nitration, and it creates nitrobenzene. Nitric acid acts as the nitrating agent, while sulfuric acid acts as a dehydrating agent. This combination removes water from the reaction mixture, driving the nitration reaction forward.
Next, you reduce nitrobenzene with iron and hydrochloric acid (HCl) to form aniline. This process is called reduction. Iron acts as a reducing agent, while hydrochloric acid acts as a catalyst and helps to provide the necessary acidic environment for the reaction to occur.
Here’s a little more about these reactions:
Nitration: The reaction between benzene and concentrated nitric acid and sulfuric acid occurs because the nitro group (-NO2) is an electron-withdrawing group. It pulls electrons away from the benzene ring, making it more reactive toward electrophilic attack. This makes the benzene ring more susceptible to attack by the nitronium ion (NO2+), which is generated by the reaction of nitric acid and sulfuric acid.
Reduction: The reduction of nitrobenzene to aniline is an important reaction because it allows us to introduce an amino group (-NH2) into the benzene ring. The amino group is a strong electron-donating group and can significantly alter the chemical and physical properties of the benzene ring.
This process is an important one in organic chemistry, as aniline is a key ingredient in the production of dyes, pharmaceuticals, and other products.
How to convert benzene to bromobenzene?
To convert benzene to bromobenzene, we need to use bromine in the presence of a Lewis acid catalyst like ferric bromide. This is a classic example of electrophilic aromatic substitution reaction. Let’s break it down:
How it works:
1. Bromine (Br2) is a relatively weak electrophile, meaning it doesn’t readily attack the electron-rich benzene ring. To make it more reactive, we need a Lewis acid catalyst like ferric bromide (FeBr3).
2. Ferric bromide reacts with bromine to form a bromonium ion (Br+). This ion is a much stronger electrophile and can readily attack the benzene ring.
3. The bromonium ion attacks the benzene ring, forming a sigma complex (an intermediate with a positive charge).
4. The sigma complex is unstable and loses a proton (H+) to regenerate the aromatic system, resulting in the formation of bromobenzene.
Here’s a simplified representation of the reaction:
Benzene + Br2 + FeBr3 → Bromobenzene + HBr
Why ferric bromide is used as a catalyst:
Ferric bromide acts as a Lewis acid and accepts a pair of electrons from the bromine molecule, weakening the Br-Br bond and making bromine more electrophilic.
* This allows the bromine to attack the benzene ring and form the bromonium ion.
Ferric bromide also helps to stabilize the sigma complex intermediate, facilitating the reaction.
Important things to remember:
* The reaction typically occurs at room temperature or slightly elevated temperatures.
Bromobenzene is a colorless liquid with a sweet odor.
* This reaction is widely used in organic chemistry for the synthesis of various aromatic compounds.
Remember, understanding the mechanism of this reaction is key to appreciating the role of each reagent and the catalyst in driving the process to form bromobenzene. Let me know if you’d like to learn more about electrophilic aromatic substitution reactions or any other organic chemistry topics!
See more here: What Are The Reactants Used In Converting Benzene To Nitrobenzene? | How To Convert Benzene To Nitrobenzene
See more new information: musicbykatie.com
How To Convert Benzene To Nitrobenzene | How To Convert Benzene Into Nitrobenzene?
How will you convert benzene into nitrobenzene? – BYJU’S
Benzene to nitrobenzene: The Nitrobenzene is prepared from Benzene by the process of nitration. That is a nitro group substitutes the hydrogen of the aromatic ring compound by reacting with nitric acid and sulphuric acid. First in the reaction, nitric acid reacts with BYJU’S
the preparation of phenylamine (aniline) – chemguide
Learn how to convert benzene to nitrobenzene by nitration and then to phenylamine by reduction. See the mechanisms, conditions, equations and practical details of the reactions. chemguide
Preparation of Phenylamine Compounds – Chemistry LibreTexts
Benzene to nitrobenzene. Benzene is nitrated by replacing one of the hydrogen atoms on the benzene ring by a nitro group, NO 2. The benzene is treated Chemistry LibreTexts
electrophilic substitution – the nitration of benzene – chemguide
Benzene is treated with a mixture of concentrated nitric acid and concentrated sulphuric acid at a temperature not exceeding 50°C. As temperature increases there is a greater chemguide
The Nitration of Benzene – Chemistry LibreTexts
This page gives you the facts and a simple, uncluttered mechanism for the electrophilic substitution reaction between benzene and a mixture of concentrated nitric Chemistry LibreTexts
How to make Nitrobenzene – YouTube
In this video i will be making nitrobenzene by the nitration of benzene.Reference: YouTube
Nitration and Sulfonation of Benzene – Chemistry LibreTexts
Nitration and sulfonation of benzene are two examples of electrophilic aromatic substitution. The nitronium ion (NO 2 +) and sulfur trioxide (SO 3) are the Chemistry LibreTexts
Nitrobenzene: Preparation, Properties, Reactions, Uses
In the laboratory, nitrobenzene is prepared by the nitration of benzene. In this process, Benzene is heated to around 60°C with a mixture of concentrated HNO 3 and scienceinfo.com
7.4.2 Nitration of Benzene – Save My Exams
Mechanism of electrophilic substitution. The nitration of benzene is one example of an electrophilic substitution reaction. A hydrogen atom is replaced by a nitro (-NO 2) group. savemyexams.com
Synthesis of substituted benzene rings I (video) | Khan Academy
The acyl group must come on before the nitro group, which means in this step, we’re going to put on the nitro group. So the immediate precursor to this molecule– we Khan Academy
How Will You Convert Benzene Into (A) P-Nitrobromobenzene (B) M-Nitrobromobenzene
How To Convert Benzene Into Nitrobenzene…. Aromatic Conversion
Benzene To Nitrobenzene | Benzene Se Nitrobenzene | Organic Chemistry Conversion
Benzene To Nitrobenzene | Conversion Benzene To Nitrobenzene | Nitration Of Benzene
How To Make Nitrobenzene
How Will You Convert Benzene Into P-Nitrobromobenzene, Etc Chemistry
How To Make Benzene
Turning Vegetable Oil Into Nitroglycerin
Link to this article: how to convert benzene to nitrobenzene.
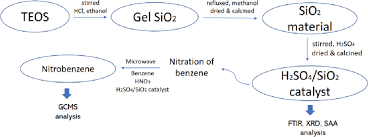
See more articles in the same category here: https://musicbykatie.com/wiki-how/
