Table of Contents
Which compounds undergo hell Volhard Zelinsky reaction?
Propionic acid is a prime example of a compound that readily undergoes this reaction. The HVZ reaction is a powerful tool in organic chemistry, allowing us to selectively introduce a halogen atom at the alpha carbon of a carboxylic acid. This reaction relies on the presence of a halogen (like bromine or chlorine) and a catalyst, usually red phosphorus.
But why is propionic acid so amenable to the HVZ reaction? It boils down to the structure of the molecule. Propionic acid has a methyl group (CH3) attached to the alpha carbon. This methyl group provides the necessary electron density to stabilize the intermediate formed during the reaction. Think of it like a stabilizing handrail for a climber, making the reaction pathway smoother.
Now, let’s unpack what makes the HVZ reaction so special. The reaction proceeds through a series of steps, starting with the formation of an acyl halide. This acyl halide is then attacked by a halogen molecule, leading to the formation of an alpha-halo carboxylic acid. The red phosphorus plays a crucial role by reacting with the halogen to generate a small amount of phosphorus trihalide. This trihalide then acts as the catalyst, facilitating the reaction by forming the acyl halide.
But what about other compounds? Not every carboxylic acid can participate in the HVZ reaction. The key factor is the presence of at least one hydrogen atom on the alpha carbon. If the alpha carbon is substituted with a bulky group, the reaction won’t proceed. This is because the bulky group hinders the attack of the halogen molecule on the acyl halide.
The HVZ reaction is a beautiful example of how subtle structural features can have a profound impact on the reactivity of organic molecules. Understanding these nuances can help us predict which compounds will react and design new synthetic strategies.
Which of the following acids does not undergo the Hell Volhard Zelinsky reaction?
The HVZ reaction is all about introducing a halogen (like bromine) to the alpha carbon of a carboxylic acid. This alpha carbon is the one directly attached to the carboxyl group (-COOH). For this reaction to happen, you need at least one hydrogen atom attached to this alpha carbon.
Now, look at 2,2-dimethylpropanoic acid. The alpha carbon has two methyl groups attached to it – those are CH3 groups. There are no hydrogens directly attached to the alpha carbon.
Think of it like this: the HVZ reaction needs a spot to attach the halogen, and that spot needs to have a hydrogen. 2,2-dimethylpropanoic acid doesn’t offer that spot because it’s all filled up with methyl groups.
Here’s a little more about alpha hydrogens and the HVZ reaction:
What are alpha hydrogens? These are the hydrogen atoms attached to the carbon atom next to the carboxyl group (the one we call the alpha carbon).
Why are alpha hydrogens important? They are crucial for the HVZ reaction because they’re what the halogen replaces. It’s like a hydrogen atom being swapped out for a halogen atom.
How does the HVZ reaction work? The reaction uses a mixture of phosphorus tribromide (PBr3) and bromine (Br2) to introduce the bromine to the alpha carbon. The mechanism involves several steps, but the key is that the bromine replaces an alpha hydrogen.
The HVZ reaction is an important tool in organic chemistry, allowing us to add halogens to carboxylic acids. Understanding the requirement for alpha hydrogens helps us predict which acids will participate in the reaction and which will not.
Which carboxylic acid reacts with Br2 and red phosphorus?
Let’s break it down:
Carboxylic acids with α-hydrogens: The key is that the carboxylic acid needs a hydrogen atom attached to the carbon next to the carboxyl group (COOH). These are called α-hydrogens. This is where the bromine will be added.
Bromine (Br2) and Red Phosphorus: The bromine acts as the halogenating agent. Red phosphorus is used to generate a small amount of phosphorus tribromide (PBr3), which is crucial for the reaction.
α-halocarboxylic acids: The product is a carboxylic acid with a bromine atom attached to the carbon next to the carboxyl group, creating an α-halocarboxylic acid.
Think of it like this: The reaction essentially replaces an α-hydrogen with a bromine atom.
Let’s look at an example:
Acetic acid (CH3COOH) has an α-hydrogen, so it will react with Br2 and red phosphorus to produce α-bromoacetic acid (BrCH2COOH).
The mechanism of the Hell-Volhard-Zelinsky reaction is interesting:
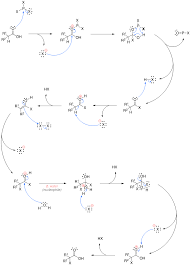
1. Formation of PBr3: Red phosphorus reacts with bromine to generate phosphorus tribromide (PBr3).
2. Reaction with the Carboxylic Acid: PBr3 reacts with the carboxylic acid to form an acyl bromide.
3. α-Bromination: The acyl bromide then undergoes bromination at the α-position, forming the α-bromoacyl bromide.
4. Hydrolysis: The α-bromoacyl bromide is hydrolyzed to produce the final product, the α-halocarboxylic acid.
Why is the Hell-Volhard-Zelinsky reaction important?
Synthesis of useful compounds: It’s a key method for synthesizing a variety of useful compounds, including α-halocarboxylic acids, which are important intermediates in organic synthesis.
Selective bromination: The reaction is highly selective, meaning it primarily brominates at the α-position, making it a valuable tool in organic chemistry.
In conclusion, the Hell-Volhard-Zelinsky reaction is a powerful tool for introducing bromine at the α-position of carboxylic acids with α-hydrogens, leading to the synthesis of various useful α-halocarboxylic acids.
What does Br2 do to an alkyne?
Br2, a bromine molecule, approaches the alkyne and gets polarized. This happens because the alkyne’s triple bond, with its π electrons, is attracted to the bromine. The π electrons attack the polarized bromine, creating a C-Br bond and releasing a bromide ion.
This reaction is called halogenation. It’s a powerful way to add bromine atoms to the alkyne, transforming it into a new molecule. The bromine atoms attach to the alkyne’s carbon atoms, changing the structure and properties of the alkyne.
But what makes Br2 so special for this reaction? It’s all about its polarizability. The bromine atoms in Br2 can easily shift their electron cloud, creating a temporary positive and negative end. This temporary difference in charge is what allows the alkyne to attack the bromine and form the new C-Br bond.
Here’s a visual way to think about it:
* Imagine Br2 as a slightly wobbly pair of dumbbells. The electrons in the molecule aren’t evenly distributed, creating a moment where one end of the dumbbell is slightly more positive and the other end is slightly more negative.
* The alkyne, with its cloud of π electrons, is like a magnet. It’s attracted to the positive end of the Br2 “dumbbell.” This attraction triggers the attack, forming a new bond and changing the structure of the alkyne.
Remember, this halogenation reaction is a key step in many organic synthesis reactions. It’s the first stage in building more complex molecules and making new materials.
Why doesn’t formic acid undergo the HVZ reaction?
Let’s break down why the α-hydrogen is so important for the HVZ reaction to work:
1. The Reaction Mechanism: The HVZ reaction starts with the α-hydrogen being removed by a bromine atom. This produces an α-bromoacid which is the final product.
2. Formic Acid’s Structure: Formic acid (HCOOH) has a single carbon atom connected to a hydroxyl group (OH) and a carbonyl group (C=O). The α-carbon is the carbonyl carbon itself. This means there are no α-hydrogens present.
Therefore, because formic acid lacks an α-hydrogen, it cannot participate in the HVZ reaction. You can think of it like a puzzle piece – the HVZ reaction needs that α-hydrogen to fit together and complete the reaction. Without it, the puzzle is incomplete.
Why is carboxylic acid stronger than phenol?
It all boils down to the stability of the conjugate bases formed when these acids lose a proton. In carboxylic acids, the negative charge on the carboxylate ion is spread out over two oxygen atoms, which are highly electronegative. This delocalization of negative charge makes the carboxylate ion very stable.
On the other hand, in phenols, the negative charge on the phenoxide ion is primarily localized on the oxygen atom. It’s delocalized to a lesser extent across the benzene ring because the carbon atoms are less electronegative than oxygen. This makes the phenoxide ion less stable compared to the carboxylate ion.
Think of it this way: The more stable the conjugate base, the more likely the acid will lose a proton. Since the carboxylate ion is more stable than the phenoxide ion, carboxylic acids are more willing to donate a proton, making them stronger acids than phenols.
Let me give you a little more detail to clarify. The delocalization of the negative charge in the carboxylate ion happens through resonance. We can draw two resonance structures for the carboxylate ion, where the double bond alternates between the carbon and one of the oxygen atoms. This means the negative charge is shared equally between the two oxygen atoms, making the carboxylate ion very stable.
In the phenoxide ion, the negative charge on the oxygen atom can be delocalized into the benzene ring through resonance. However, the delocalization is not as effective because the carbon atoms in the benzene ring are less electronegative than oxygen. This means the negative charge is concentrated more on the oxygen atom, making the phenoxide ion less stable.
Therefore, the greater stability of the carboxylate ion compared to the phenoxide ion is the key reason why carboxylic acids are stronger acids than phenols.
How to reduce carboxylic acid?
You can easily transform carboxylic acids into alcohols using strong reducing agents like lithium aluminum hydride (LiAlH4). This powerful reagent can also handle other related compounds like acid halides and esters, turning them into alcohols as well. Amides, on the other hand, are reduced to amines using the same reagent.
Lithium aluminum hydride (LiAlH4) is a very strong reducing agent that’s often used in organic chemistry. It’s known for its ability to break strong carbon-oxygen bonds, which is why it’s so effective in reducing carboxylic acids, acid halides, and esters to alcohols.
Here’s a breakdown of what happens during the reduction process:
Carboxylic acids react with LiAlH4 to form an intermediate aluminum alkoxide. This intermediate is then hydrolyzed (reacted with water) to yield the corresponding alcohol.
Acid halides and esters undergo similar reactions, with the LiAlH4 breaking the carbon-halogen or carbon-oxygen bond to form an alcohol.
Amides are a bit different. They react with LiAlH4 to form an amine, where the nitrogen atom replaces the oxygen atom.
Important things to remember about LiAlH4:
* It’s very reactive and must be handled with care.
* It’s typically used in anhydrous (water-free) conditions because it reacts violently with water.
* The reaction is usually carried out in an ether solvent, like diethyl ether or tetrahydrofuran (THF).
Why use LiAlH4?
While there are other reducing agents available, LiAlH4 is often preferred because it’s highly effective in reducing a wide range of carbonyl compounds, including carboxylic acids. It’s a versatile reagent that can be used to synthesize a variety of important organic compounds.
Let me know if you’d like to explore other ways to reduce carboxylic acids or learn more about specific reaction mechanisms!
See more here: What Does Br2 Do To Carboxylic Acid? | Hell Volhard Zelinsky Reaction Amino Acid
What is Hell Volhard Zelinsky chlorination?
The Hell-Volhard-Zelinsky (HVZ) reaction is a useful method for converting carboxylic acids into alpha-halo acids. This reaction is named after the three chemists who discovered it: Carl Magnus von Hell, Jacob Volhard, and Nikolai Zelinsky.
The HVZ reaction requires the presence of alpha hydrogen atoms on the carboxylic acid. Alpha hydrogen atoms are the hydrogen atoms directly attached to the carbon atom next to the carboxyl group. Carboxylic acids that lack these alpha hydrogens cannot undergo the HVZ reaction.
So, what happens during HVZ chlorination?
Firstly, we add phosphorus and halogen, typically bromine or chlorine, to the carboxylic acid. Secondly, the phosphorus reacts with the halogen to form phosphorus halides. These phosphorus halides act as the electrophilic reagent in the reaction. Thirdly, the alpha hydrogen is removed as a proton by the phosphorus halide. This leaves a carbanion which is then attacked by the electrophilic halogen, resulting in the formation of the alpha-halo acid.
The HVZ reaction is often used to synthesize alpha-bromo acids, which are valuable intermediates in the synthesis of various organic compounds. For example, alpha-bromo acids can be used to synthesize alpha-amino acids, which are the building blocks of proteins.
Let’s take a closer look at alpha-halo acids. These are carboxylic acids with a halogen atom attached to the carbon atom adjacent to the carboxyl group. They’re often involved in further chemical reactions. For example, they can undergo nucleophilic substitution to form alpha-hydroxy acids, which are commonly found in fruits and vegetables.
You may also find that the HVZ reaction is used in the synthesis of alpha-keto acids, which are important intermediates in the citric acid cycle.
In summary, the HVZ reaction is a useful and versatile tool in organic chemistry. It allows us to convert carboxylic acids into a range of valuable compounds, including alpha-halo acids, alpha-amino acids, alpha-hydroxy acids, and alpha-keto acids.
Who invented the Hell-Volhard-Zelinsky reaction?
Carl Magnus von Hell was the first to report a version of this reaction in 1881. His work focused on the alpha-bromination of carboxylic acids using phosphorus tribromide. This was a significant breakthrough, as it provided a new method for introducing bromine atoms into organic molecules.
Jacob Volhard further expanded on Hell’s work by introducing a modified procedure that used red phosphorus and bromine. This method, now known as the Hell-Volhard-Zelinsky reaction, became a standard procedure for alpha-bromination. Volhard’s contribution was crucial in refining the reaction and making it more practical for synthesis.
Nikolai Dmitrievich Zelinsky later extended the scope of the reaction by demonstrating that it could be applied to a wider range of carboxylic acids. Zelinsky’s work further solidified the importance of this reaction in organic chemistry.
Therefore, the Hell-Volhard-Zelinsky reaction is named after these three scientists who each contributed significantly to its development and understanding. Their collective efforts led to a powerful tool for synthesizing a wide range of important organic compounds.
See more new information: musicbykatie.com
Hell Volhard Zelinsky Reaction Amino Acid | Which Carboxylic Acid Gives The Hell Volhard-Zelinsky Reaction?
Hey there, chemistry enthusiasts! Today, we’re diving into a classic organic chemistry reaction: the Hell-Volhard-Zelinsky (HVZ) reaction. This powerful technique allows us to introduce alpha-halo groups to carboxylic acids. It’s a super useful tool, particularly in the realm of amino acid synthesis, and we’re going to explore exactly how it works.
Understanding the Basics
The HVZ reaction involves treating a carboxylic acid with phosphorus tribromide (PBr3) or phosphorus trichloride (PCl3), followed by bromine (Br2) or chlorine (Cl2). This process ultimately converts the alpha-hydrogen of the carboxylic acid into a halo group.
Let’s break it down step by step:
1. Activation: The first step involves the reaction of the carboxylic acid with phosphorus tribromide (PBr3) or phosphorus trichloride (PCl3). This creates an acyl halide.
2. Alpha-Halogenation: The acyl halide is then treated with bromine (Br2) or chlorine (Cl2) in the presence of a catalytic amount of red phosphorus. The alpha-hydrogen is then replaced by a halo group.
Why does this reaction happen? Well, the alpha-hydrogen in the acyl halide is significantly more acidic than the hydrogen in the original carboxylic acid. This enhanced acidity makes the alpha-hydrogen more susceptible to halogenation.
The Role of the HVZ Reaction in Amino Acid Synthesis
Now, why are we talking about the HVZ reaction in the context of amino acid synthesis? You might be wondering, “What’s the connection?”
The HVZ reaction plays a crucial role in synthesizing alpha-amino acids, the building blocks of proteins. Here’s how:
1. Starting with a Simple Carboxylic Acid: We begin with a simple carboxylic acid, like acetic acid. This acid acts as our starting point for the amino acid synthesis.
2. Introducing the Halo Group: We then use the HVZ reaction to introduce a halo group at the alpha position. This creates an alpha-halo carboxylic acid.
3. Nucleophilic Substitution: Next, we use a nucleophile like ammonia (NH3) to displace the halo group. This creates an amino acid.
4. Final Touches: The final step involves adjusting the side chain to create a specific amino acid. This might involve additional reactions to introduce the desired functional groups.
Key Applications of the HVZ Reaction
The HVZ reaction has numerous applications in organic chemistry, beyond amino acid synthesis. Here are some of its key uses:
Synthesis of Alpha-Halo Acids: As we’ve discussed, the HVZ reaction is a powerful tool for synthesizing alpha-halo acids. These compounds are versatile building blocks in organic synthesis, often used to synthesize other important molecules.
Preparation of Chiral Compounds: The HVZ reaction can also be used to prepare chiral compounds. This is because the alpha-carbon becomes stereogenic after the halogenation step.
Drug Synthesis: The HVZ reaction has found applications in the synthesis of several pharmaceutical compounds. This highlights its importance in the pharmaceutical industry.
Considerations for Successful HVZ Reactions
While the HVZ reaction is a powerful tool, it’s important to consider certain aspects for successful implementation:
Choice of Halogen: The choice of halogen can affect the reaction’s outcome. Generally, bromine is preferred over chlorine due to its higher reactivity.
Reaction Conditions: The reaction conditions are crucial. The reaction should be carried out in the presence of a catalytic amount of red phosphorus to prevent the formation of unwanted side products.
Workup: The workup after the reaction is also important. It involves removing the excess phosphorus tribromide/phosphorus trichloride and other byproducts.
Safety Precautions: The HVZ reaction involves using halogens, which are highly reactive and corrosive. It’s crucial to take appropriate safety precautions when carrying out this reaction.
The Bottom Line
The Hell-Volhard-Zelinsky (HVZ) reaction is a versatile tool in organic chemistry. It provides a convenient way to introduce alpha-halo groups to carboxylic acids, which is especially important in amino acid synthesis. By understanding the mechanism and key considerations, you can harness the power of the HVZ reaction for your synthetic endeavors.
FAQs About the Hell-Volhard-Zelinsky Reaction
Here are some frequently asked questions about the HVZ reaction:
Q: What is the mechanism of the HVZ reaction?
A: The HVZ reaction proceeds through a series of steps:
1. Formation of the Acyl Halide: The carboxylic acid reacts with phosphorus tribromide (PBr3) or phosphorus trichloride (PCl3) to form the acyl halide.
2. Alpha-Halogenation: The acyl halide then reacts with bromine (Br2) or chlorine (Cl2) in the presence of a catalytic amount of red phosphorus to form the alpha-halo acyl halide.
3. Hydrolysis: The alpha-halo acyl halide is then hydrolyzed to give the alpha-halo carboxylic acid.
Q: Why is the HVZ reaction used in amino acid synthesis?
A: The HVZ reaction is used in amino acid synthesis because it allows for the introduction of a halo group at the alpha position of a carboxylic acid. This halo group can then be displaced by a nucleophile like ammonia (NH3) to form the amino acid.
Q: What are the limitations of the HVZ reaction?
A: The HVZ reaction has some limitations, including:
Reactivity of the Carboxylic Acid: The HVZ reaction is only suitable for carboxylic acids with alpha-hydrogens.
Side Reactions: The HVZ reaction can be prone to side reactions, such as over-halogenation.
Safety: The HVZ reaction involves the use of halogens, which are highly reactive and corrosive.
Q: Are there any alternative methods for synthesizing alpha-halo acids?
A: Yes, there are other methods for synthesizing alpha-halo acids, including:
Halogenation of Ketones: Ketones can be halogenated at the alpha position using halogens like bromine (Br2) or chlorine (Cl2). The resulting alpha-halo ketones can then be converted to alpha-halo acids through oxidation.
Use of N-Bromosuccinimide (NBS):NBS is a reagent that can be used to selectively brominate the alpha position of carboxylic acids in the presence of light.
Q: What are some practical applications of the HVZ reaction?
A: The HVZ reaction has found numerous practical applications, including:
Synthesis of Pharmaceuticals: The HVZ reaction is used in the synthesis of several pharmaceutical compounds, including antibiotics, anti-inflammatory agents, and anti-cancer drugs.
Preparation of Chiral Compounds: The HVZ reaction can be used to prepare chiral compounds, which are important in pharmaceutical synthesis and other fields.
Organic Synthesis: The HVZ reaction is a valuable tool for organic synthesis, allowing chemists to synthesize a wide variety of molecules.
Q: How can I learn more about the HVZ reaction?
A: If you’re interested in learning more about the HVZ reaction, there are several resources available:
Organic Chemistry Textbooks: Many organic chemistry textbooks cover the HVZ reaction in detail.
Online Resources: There are numerous websites and online articles that discuss the HVZ reaction.
Scientific Journals: You can find research articles on the HVZ reaction in scientific journals.
Chemistry Courses: Many chemistry courses cover the HVZ reaction.
Understanding the HVZ reaction is an essential part of your organic chemistry journey. So, get out there, experiment, and explore the fascinating world of chemical reactions!
Hell-Volhard-Zelinsky reaction – Chemistry LibreTexts
The Hell Volhard Zelinsky reaction demonstrates a method for alpha addition with a carboxylic acid. The gist of the method is to Chemistry LibreTexts
Hell-Volhard-Zelinsky Reaction – Organic Chemistry
Mechanism of the Hell-Volhard-Zelinsky Reaction. Phosphorus reacts with bromine to give phosphorus tribromide, and in the first step this converts the carboxylic acid into an acyl bromide. An acyl bromide can Organic Chemistry Portal
Hell-Volhard-Zelinsky Reaction – Chemistry Learner
Hell-Volhard-Zelinsky reaction is used to convert phenylacetic acid to 2-bromo-2-phenylacetic acid when reacted with PBr 3. HVZ reaction is also used to prepare alanine, an α-amino acid, after the ammonolysis of 2 Chemistry Learner
Amino Acid Synthesis and Protection Reactions – OrgoSolver
One method involves using the Hell-Volhard-Zelinsky (HVZ) reaction in combination with the Gabriel synthesis, which involves the transformation of alpha-halo acids or esters OrgoSolver
Hell-Volhard-Zelinsky (HVZ) Reaction
Hell-Volhard-Zelinsky (HVZ) Reaction. The Hell-Volhard-Zelinsky reaction effects the synthesis of α-halogenated carboxylic acids. These are useful synthetic Pomona College
Hell volhard zelinsky reaction, mechanism and examples
Hell Volhard Zelinsky is a reaction between aliphatic acids having at least one alpha-hydrogen and bromine in presence of phosphorus and phosphorus tribromide chemistnotes.com
Hell-Volhard-Zelinskii Reaction – Chemistry LibreTexts
However, carboxylic acids, can be brominated in the alpha position with a mixture of Br 2 and PBr 3 in a reaction called the Hell-Volhard-Zelinskii reaction. The Chemistry LibreTexts
Hell-Volhard-Zelinsky Reaction | Chem-Station Int. Ed.
General Characteristics. The classical method to convert carboxylic acids into α-haloacyl halides using phosphorus (III) halide is known as the Hell-Volhard-Zelinsky Chem-Station Int. Ed.
Hell-Volhard-Zelinksy Rxn And Mechanism
Understanding The Significance And Mechanism Of The Hell-Volhard-Zelinsky Reaction
Hell-Volhard-Zelinsky Reaction
Question-Answer (Part-1) Synthesis Of Amino Acids, Hell-Volhard-Zelinsky (Hvz Reaction) Iit Jam Net
20: The Hell-Volhard-Zelinski Reaction
21.3B The Hvz Reaction
Hell–Volhard–Zelinsky Reaction
Hell-Volhard-Zelinsky Reaction
Link to this article: hell volhard zelinsky reaction amino acid.
See more articles in the same category here: https://musicbykatie.com/wiki-how/
