Table of Contents
What happens to reactants during a reaction?
During a chemical reaction, the atoms within the reactants rearrange themselves and form new bonds. This results in the creation of one or more products with different characteristics compared to the original reactants. This transformation is known as a chemical change.
Let’s think about it like this: Imagine you have a bunch of Lego blocks (our reactants). You can build different structures with those blocks (our products) by rearranging them and connecting them in different ways.
The key takeaway is that the atoms themselves don’t disappear or get destroyed during a chemical reaction. They simply change their arrangement and how they are connected.
Here’s a deeper dive into what happens to reactants on a molecular level. When reactants collide with enough energy, their bonds can break. This energy, often called activation energy, is necessary to initiate the reaction. Once the bonds break, atoms can rearrange and form new bonds, creating the products.
This process of breaking and forming bonds is constantly happening in chemical reactions. It’s a dynamic dance of atoms rearranging to create something new.
Let’s take a simple example: the reaction of hydrogen and oxygen to form water. In this reaction, hydrogen molecules (H2) and oxygen molecules (O2) collide with enough energy to break their existing bonds. The hydrogen and oxygen atoms then rearrange and form new bonds, creating water molecules (H2O). The water molecules have different properties than the original hydrogen and oxygen molecules.
Remember, chemical reactions are all about rearranging atoms and forming new bonds. It’s like building something new with the same set of building blocks!
What always happens during a chemical reaction?
In every chemical change, the atoms in the reactants get rearranged and form new bonds with each other. This creates one or more new products with entirely different properties than the original reactants. This rearranging of atoms is what makes a chemical change different from a physical change. Think of it like building with LEGOs – you can take the same blocks and create different structures, and that’s exactly what happens in a chemical reaction!
Let’s break it down a bit more. Imagine you have two ingredients: hydrogen and oxygen. These are the reactants. When they react, they form water. That’s the product. Hydrogen and oxygen are gases, but water is a liquid. That’s a big difference! This is because the atoms of hydrogen and oxygen bonded in a new way to form water, creating a completely different substance with new properties.
It’s like playing with building blocks, except that instead of plastic blocks, we’re talking about the tiny, invisible building blocks of everything – atoms! In a chemical reaction, the atoms of the reactants are rearranged to form new products with different properties. This change in the arrangement of atoms is what defines a chemical change.
What is a reactant in a chemical reaction?
Reactants are the starting materials that go through a chemical change to create a product. Think of them as the ingredients you mix together to bake a cake. The cake, in this case, is the product.
Now, reactants won’t just magically transform into a product. They need the right conditions to react. Imagine trying to bake a cake without an oven—it won’t work! Similarly, reactants need the right temperature, pressure, or even the presence of a catalyst to initiate the chemical reaction.
To illustrate this further, let’s look at a simple example:
Reactant 1: Hydrogen (H2)
Reactant 2: Oxygen (O2)
Product: Water (H2O)
The chemical equation for this reaction is:
2H2 + O2 -> 2H2O
This equation tells us that two molecules of hydrogen react with one molecule of oxygen to form two molecules of water.
Here’s what’s important to remember about reactants:
* They are consumed during the reaction.
* They are written on the left side of the chemical equation.
* They are the substances that change during the reaction.
* They are the foundation for the creation of new substances, which are the products.
Understanding reactants is crucial to grasping the fundamentals of chemistry. They are the building blocks of chemical reactions, and without them, there would be no change or transformation. So, the next time you see a chemical equation, remember the reactants are the key players in this chemical dance!
Are reactants always negative?
Think of it like this: You’re driving a car. The reactants are like the gas in your tank. As you drive, the amount of gas in your tank decreases. The reaction rate is how fast you’re using up the gas. The change in concentration of the gas is negative because it’s decreasing. We add the negative sign in front of the change in concentration to ensure that the reaction rate is always positive.
Here’s a breakdown of the key concepts:
Reactants: These are the substances that are consumed during a chemical reaction. As the reaction proceeds, the concentration of reactants decreases.
Products: These are the substances that are formed during a chemical reaction. As the reaction proceeds, the concentration of products increases.
Reaction Rate: This is a measure of how fast a chemical reaction is proceeding. It’s typically defined as the change in concentration of a reactant or product over time.
Change in Concentration: This is the difference between the initial concentration of a substance and its concentration at a later time.
When calculating the reaction rate, we are interested in the rate of change of concentration of reactants or products. Since the concentration of reactants decreases over time, the change in concentration is negative. We use the negative sign to ensure that the reaction rate is always positive. This makes the reaction rate a more meaningful measure, as it indicates how fast the reaction is proceeding, regardless of whether the concentration of the substance is increasing or decreasing.
Do reactants always react together?
The products of a reaction are influenced by multiple factors, not just the reactants themselves. Temperature, pressure, and the presence of a catalyst all play significant roles in determining the reaction’s course. For example, increasing the temperature can provide the energy needed for a reaction to occur, while a catalyst can speed up the reaction without being consumed.
Imagine a chemical reaction as a recipe. You might have the same ingredients (reactants), but depending on the oven temperature (temperature), the cooking time (pressure), and any special tools you use (catalyst), you’ll get different outcomes.
To understand this more deeply, let’s delve into the world of reaction kinetics and thermodynamics. Reaction kinetics studies the rate of a reaction, while thermodynamics focuses on the energy changes involved.
Reaction kinetics explains why some reactions are fast, like the combustion of gasoline, while others are slow, like the rusting of iron. The rate of a reaction can be influenced by factors like concentration of reactants, surface area, and temperature.
Thermodynamics helps us predict whether a reaction will occur spontaneously or not. The key concept here is Gibbs free energy, which tells us whether a reaction will release or absorb energy. A negative Gibbs free energy indicates a spontaneous reaction, meaning it will occur naturally without the need for external energy input.
Let’s consider a simple example: the reaction between sodium and chlorine. This reaction is spontaneous because it releases energy, but it needs a specific set of conditions to occur. You wouldn’t see sodium and chlorine spontaneously reacting in a room at room temperature. However, if you heat the mixture or introduce a catalyst, the reaction will proceed.
So, while reactants might have the potential to react, the actual outcome is determined by a complex interplay of factors. Understanding these factors is crucial for predicting and controlling chemical reactions.
What happens to compounds during a chemical reaction?
During a reaction, the bonds between atoms in the reactants get broken. Think of it like taking apart a Lego set. Once those bonds are broken, the atoms or pieces of molecules can rearrange themselves and form new bonds to create the products. It’s like building a new Lego creation with the same pieces!
This process of breaking and forming bonds involves energy. You need energy to break bonds, kind of like how you need energy to pull apart those stubborn Lego pieces. And when new bonds are formed, energy is released. It’s like the satisfying click you hear when you connect two Lego bricks!
Here’s a more detailed explanation:
Breaking Bonds: When bonds are broken, it requires energy input. This energy can come from various sources, such as heat, light, or electricity. Think of it like heating up a Lego set to make the pieces easier to separate.
Forming Bonds: On the other hand, when new bonds are formed, energy is released. This release of energy can be in the form of heat, light, or sound. Think of it like how you feel a little warmth from the friction of connecting those Lego bricks.
The net energy change in a chemical reaction depends on the difference between the energy required to break bonds and the energy released when new bonds are formed. If more energy is released than absorbed, the reaction is exothermic, meaning it releases heat. If more energy is absorbed than released, the reaction is endothermic, meaning it absorbs heat.
Understanding how bonds are broken and formed is crucial to understanding the essence of chemical reactions. It’s like knowing the secret to building the coolest Lego creations – you need to know how to take things apart before you can put them back together in a new way!
When a chemical reaction occurs _____?
Think of it like baking a cake. The reactants are the ingredients you start with: flour, sugar, eggs, butter, etc. The products are the cake itself. The cake has a completely different taste, texture, and appearance than the individual ingredients. This is because the chemical bonds between the atoms in the ingredients have been rearranged during the baking process.
Let’s look at a simple example: the reaction between hydrogen (H2) and oxygen (O2) to form water (H2O). Hydrogen and oxygen are gases at room temperature, while water is a liquid. This change in state is a clear indication that a chemical reaction has occurred.
Here’s another example: rusting. When iron is exposed to air and water, it reacts with oxygen to form iron oxide. Iron oxide is a reddish-brown solid that is very different from the shiny gray metal we call iron. The formation of rust is a chemical reaction.
Chemical reactions are everywhere in our lives, from the burning of fuel to the growth of plants. They are responsible for everything from the food we eat to the air we breathe. Understanding how chemical reactions work is essential for understanding the world around us.
What does a chemical reaction always result in?
Think about baking a cake. You mix flour, sugar, eggs, and other ingredients. You then apply heat, which causes the ingredients to react chemically. The result? A cake! The cake is a completely different substance than the individual ingredients you started with. It has new properties, like a delicious taste and a spongy texture. The chemical reaction that created the cake is irreversible—you can’t simply unbake the cake to get the original ingredients back.
However, some chemical reactions are reversible. Think about dissolving sugar in water. The sugar dissolves, forming a sugar solution. This is a chemical reaction. But you can reverse it by heating the solution, causing the water to evaporate and leaving behind the solid sugar crystals.
The key takeaway is that chemical reactions always involve the formation of new substances with different properties than the original substances. Sometimes these changes can be reversed, but often they are permanent.
See more here: What Always Happens During A Chemical Reaction? | During A Chemical Reaction Reactants Always
What is a chemical reaction?
Essentially, a chemical reaction is like a recipe where you mix ingredients (reactants) and transform them into something new (products). Imagine baking a cake. You combine flour, sugar, eggs, and other ingredients, and through the process of baking, they change into a delicious cake.
The key to understanding chemical reactions is that atoms are rearranged. In our cake example, the atoms from the flour, sugar, and eggs are rearranged to create the cake’s molecules. This rearrangement involves chemical bonds – the forces that hold atoms together.
During a chemical reaction, existing chemical bonds break, and new ones form. Think of it like building with LEGOs. You take apart existing structures and rebuild them in a different way.
We see chemical reactions happening all around us. From rusting iron to burning wood, these are all examples of chemical reactions where the substances involved transform into something else.
Let’s explore some specific types of chemical reactions:
Synthesis reactions: These are like building something new. Two or more simple substances combine to form a more complex substance. For example, when you combine hydrogen and oxygen, you create water.
Decomposition reactions: These are the opposite of synthesis reactions. You break down a complex substance into simpler ones. When you heat limestone, it breaks down into calcium oxide and carbon dioxide.
Replacement reactions: Think of this like a swap. One element replaces another in a compound. For example, when iron reacts with copper sulfate, iron replaces copper to form iron sulfate.
Combustion reactions: This is a chemical reaction that produces heat and light. A common example is burning wood or gas, where the fuel reacts with oxygen to produce carbon dioxide, water, and heat.
Understanding chemical reactions gives us a deeper understanding of the world around us, from how our bodies function to how we create new materials and technologies.
What happens during a chemical reaction?
It’s all about chemical changes. Imagine atoms as tiny building blocks, and chemical bonds are like the glue holding them together. During a reaction, some of this glue breaks, and new glue forms, rearranging the blocks!
Reactants are like the ingredients you start with, and products are the new things you create. Think of baking a cake: flour, sugar, and eggs are the reactants, and the cake is the product. The cool thing is, the atoms themselves don’t disappear; they just get rearranged into a new arrangement.
Here’s a simple way to think about it: Imagine you have a pile of LEGO bricks. You can build different things with the same bricks, just by changing how they’re connected. That’s essentially what happens in a chemical reaction!
Let’s delve deeper into the world of chemical bonds: They are the forces that hold atoms together. Think of them like tiny magnets. There are two main types of bonds:
Ionic Bonds: These bonds happen when one atom gives up an electron to another atom, creating a positive and a negative charge. Imagine two magnets, one north and one south, pulling each other together.
Covalent Bonds: This is when atoms share electrons. It’s like two magnets, each with a north and south pole, sticking together because they share a common pole.
When a chemical reaction occurs, either these ionic or covalent bonds break, allowing the atoms to rearrange and form new bonds.
Here’s a real-world example: When you burn wood, you’re actually causing a chemical reaction. The wood (reactant) reacts with oxygen (reactant) in the air. The chemical bonds in the wood and oxygen break, and new chemical bonds form, creating carbon dioxide (product), water (product), and ash (product). This process releases energy in the form of heat and light, which is why you see flames.
So, that’s the gist of it! Chemical reactions are like a dance of atoms, where bonds break and form, creating new and exciting arrangements.
What are substances that start a chemical reaction called?
Once the reaction happens, the reactants change and form products. These are the new substances created by the reaction. It’s like baking a cake – the flour, sugar, and eggs change into a delicious cake!
You can write a chemical equation to represent a chemical reaction. The reactants are always written on the left side of the equation, and the products are on the right side. The arrow in the middle of the equation shows the direction of the reaction.
Let’s take a simple example: the reaction between hydrogen and oxygen to form water. This is written as:
2H₂ + O₂ → 2H₂O
Here, hydrogen (H₂) and oxygen (O₂) are the reactants. They combine to form water (H₂O), which is the product.
It’s important to note that the number of atoms on each side of the equation must be the same. This is called balancing the equation, and it ensures that the reaction follows the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction.
So, the next time you see a chemical reaction, remember that the reactants are the substances that start the whole process and the products are the new substances formed as a result. It’s like a dance between molecules!
Why do reactants and products have different atoms?
Think of it like building with LEGOs. You can start with the same bricks, but build different things by putting them together in different ways. In a chemical reaction, the atoms are the LEGO bricks, and the chemical bonds are how they connect. When a reaction happens, some bonds break and new ones form. This changes the way the atoms are linked together, making a completely different molecule.
Let’s take a simple example: burning wood. Wood is mostly made of carbon, hydrogen, and oxygen. When you burn it, you’re reacting wood with oxygen from the air. The bonds in the wood break, and new bonds form with the oxygen, creating carbon dioxide (CO2) and water (H2O).
The key point is that no atoms are created or destroyed, just rearranged. So, while the reactants (wood and oxygen) have different atoms arranged in a different way than the products (carbon dioxide and water), the same number of each kind of atom is present throughout. It’s just a chemical dance where atoms are partners in different ways before and after the reaction!
See more new information: musicbykatie.com
During A Chemical Reaction, Reactants Always… Transform!
Okay, so we’re talking about chemical reactions, right? It’s like magic, but with molecules and stuff. And there’s something super important to know about these reactions – reactants always get involved. Think of it like a recipe. You need the ingredients to make the dish, just like you need reactants to make a new product in a reaction.
What are reactants? They are the substances that start the whole party. They’re the ones doing the reacting, getting all mixed up and changing their forms. And what’s the outcome? We get products, the brand new substances created by the reaction.
Now, here’s the thing: reactants always stay reactants. They’re the starting materials, the foundation of the reaction. They’re not magically transformed into something else during the process. It’s like saying the flour and sugar in your cake batter don’t become the cake itself – they’re still the key ingredients, even though they’re part of something bigger.
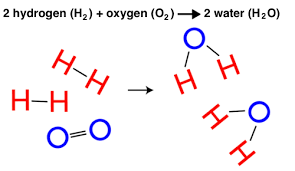
Let’s break it down further. Imagine a simple reaction: hydrogen gas (H2) and oxygen gas (O2) combining to form water (H2O).
H2 + O2 → H2O
Here, hydrogen (H2) and oxygen (O2) are our reactants. They’re the players in the reaction, and they’re the ones getting rearranged. Water (H2O) is the product, the new substance born from the reaction.
Remember, reactants always stay reactants, even though they undergo a transformation. They’re like the building blocks that create the new structure, the product, of the reaction.
The Law of Conservation of Mass
But wait, there’s a bigger picture here. We have this amazing law called the Law of Conservation of Mass. It says that in any chemical reaction, the total mass of the reactants always equals the total mass of the products. It’s like saying that in our cake batter example, the combined weight of the flour, sugar, and other ingredients is the same as the weight of the final cake.
No matter how much we mix and bake, the total amount of matter stays the same. This is because the reactants are just being rearranged, not destroyed or created. The atoms from the reactants are still there, just in a different configuration, forming the products.
Reactant Changes During a Reaction
Now, here’s a little twist. While reactants always stay reactants in terms of their role, they might change in some ways. Think about it:
Reactant amounts: The reactants might be used up as the reaction progresses. In our cake batter example, once you mix all the ingredients, the amounts of flour, sugar, etc., will decrease as they get incorporated into the cake batter.
Reactant states:Reactants can change states, like from solid to liquid or gas. Imagine ice melting into water. The ice (solid) is the reactant changing state to become water (liquid), the product.
But remember, even though the reactants may change in these ways, they’re still the reactants that start the whole process.
Key Takeaways
Reactants always start the chemical reaction.
Reactants always stay reactants, even though they might change in some ways.
* The Law of Conservation of Mass tells us that the total mass of the reactants always equals the total mass of the products.
FAQs
1. Can reactants be products?
Yes, absolutely! In a reversible reaction, the products can react to form the original reactants. It’s like a two-way street where the traffic can go in both directions.
2. What are the common types of reactants?
We have different types of reactants, but the most common ones are:
Acids: These are substances that release hydrogen ions (H+) when dissolved in water. Examples: hydrochloric acid (HCl), sulfuric acid (H2SO4)
Bases: These are substances that release hydroxide ions (OH-) when dissolved in water. Examples: sodium hydroxide (NaOH), potassium hydroxide (KOH)
Salts: These are compounds formed by the reaction of an acid and a base. Examples: sodium chloride (NaCl), potassium nitrate (KNO3)
Oxides: These are compounds that contain oxygen. Examples: carbon dioxide (CO2), iron oxide (Fe2O3)
3. How do we identify reactants in a chemical equation?
Easy peasy! Reactants are always listed on the left side of the chemical equation. They are separated by a plus sign (+) and followed by an arrow (→) that points towards the products.
4. Why are reactants important?
Reactants are the stars of the show! They’re the ingredients that make the magic happen. Without them, there’s no chemical reaction, no new products, and no chemical change.
5. Are reactants always consumed in a reaction?
Not necessarily! In some reactions, called equilibrium reactions, the reactants and products coexist in a dynamic balance. They keep reacting with each other, but the overall amounts of reactants and products stay relatively constant.
6. Can we create reactants?
That’s a tough one. We can’t create new atoms, but we can create new molecules by combining existing atoms. So, while we can’t create the basic building blocks, we can create new reactants by rearranging these atoms in different ways.
There you have it! You now have a solid understanding of reactants and their importance in chemical reactions. It’s not magic, but it’s pretty fascinating, right?
2.13: Chemical Reaction – Chemistry LibreTexts
A chemical reaction is a process in which some substances, called reactants, change into different substances, called products. During the reaction, chemical bonds break in the reactants and new chemical bonds form in the products. Chemistry LibreTexts
7: Chemical Reactions – Chemistry LibreTexts
A chemical reaction is the process in which one or more substances are changed into one or more new substances. Chemical reactions are represented by chemical equations. Chemistry LibreTexts
What Is a Chemical Reaction? Definition and Examples
A chemical reaction is a process in which the chemical structure of a substance changes, leading to the formation of a new substance with different Science Notes and Projects
Chemical reaction | Definition, Equations, Examples,
Chemists classify chemical reactions in a number of ways: by type of product, by types of reactants, by reaction outcome, and by reaction mechanism. Often a given reaction can be placed in Britannica
Chemical Reactions: Types, Definitions, and
Table Of Contents. What is a Chemical Reaction? A chemical reaction is a process in which one or more substances are converted to one or more different substances. The starting substances are called the reactants, Chemistry Learner
Lesson 6.1: What is a Chemical Reaction? – American Chemical
In a chemical reaction, reactants contact each other, bonds between atoms in the reactants are broken, and atoms rearrange and form new bonds to make American Chemical Society
Chemical Reactions Overview – Chemistry LibreTexts
Chemical reactions are the processes by which chemicals interact to form new chemicals with different compositions. Chemistry LibreTexts
Chemical reaction – Wikipedia
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction Wikipedia
Chemical Reactions | Chemistry | Visionlearning
Key concepts. Terms you should know. This is an updated version of the moduleChemical Reactions (previous version). Chemical reactions happen absolutely everywhere. visionlearning.com
Chemical Reaction And Equation/Chemical Reaction/Reactant /Chemical Equations, Exother
Chemical Reactions
Good Thinking! — Chemical Reactions In Action
What Triggers A Chemical Reaction? – Kareem Jarrah
The Law Of Conservation Of Mass – Todd Ramsey
Video Lab: Chemical Reaction: Change In Color
Why Don’T Oil And Water Mix? – John Pollard
Solving Chemical Reactions – Predicting The Products – Clear \U0026 Simple Chemistry
Link to this article: during a chemical reaction reactants always.
See more articles in the same category here: https://musicbykatie.com/wiki-how/
