Are you looking for an answer to the topic “Does temperature increase or decrease solubility in endothermic reactions?“? We answer all your questions at the website Musicbykatie.com in category: Digital Marketing Blogs You Need To Bookmark. You will find the answer right below.
In endothermic reactions, increasing the temperature increases the solubility of the solute in a solution. In exothermic reactions, increasing the temperature decreases the solubility of the solute.As temperature increases, the solubility of a solid or liquid can fluctuate depending on whether the dissolution reaction is exothermic or endothermic. In endothermic dissolution reactions, the net energy from breaking and forming bonds results in heat energy being absorbed into the system as the solute dissolves.Increasing temperature introduces more heat into the system. According to Le Chatelier’s Principle, the system will adjust to this excess heat energy by inhibiting the dissolution reaction. Increasing temperature, therefore, decreases the solubility of the solute.

Table of Contents
How does temperature affect solubility in an endothermic reaction?
As temperature increases, the solubility of a solid or liquid can fluctuate depending on whether the dissolution reaction is exothermic or endothermic. In endothermic dissolution reactions, the net energy from breaking and forming bonds results in heat energy being absorbed into the system as the solute dissolves.
Does temperature increase or decrease solubility in exothermic reactions?
Increasing temperature introduces more heat into the system. According to Le Chatelier’s Principle, the system will adjust to this excess heat energy by inhibiting the dissolution reaction. Increasing temperature, therefore, decreases the solubility of the solute.
What Are Endothermic Exothermic Reactions | Chemistry | FuseSchool
Images related to the topicWhat Are Endothermic Exothermic Reactions | Chemistry | FuseSchool

Does temperature increase or decrease solubility?
Therefore, the solubility (concentration) increases with an increase in temperature. If the process is exothermic (heat given off). A temperature rise will decrease the solubility by shifting the equilibrium to the left.
What happens when temperature increases in endothermic?
If the reaction is endothermic as written, an increase in temperature will cause the forward reaction to occur, increasing the amounts of the products and decreasing the amounts of reactants. Lowering the temperature will produce the opposite response. A change of temperature has no effect on an athermal reaction.
Why does solubility increase in endothermic reactions?
For many solids that dissolve in liquid, the solubility increases with the increase in temperature. Increase in temperature increases kinetic energy that comes with higher temperature allowing the solvent molecules to more efficiently break apart the solute molecules that are held by strong intermolecular attraction.
How does temperature affect solubility?
Key Points. For many solids dissolved in liquid water, the solubility increases with temperature. The increase in kinetic energy that comes with higher temperatures allows the solvent molecules to more effectively break apart the solute molecules that are held together by intermolecular attractions.
Why does solubility decrease in exothermic?
In endothermic reactions, increasing the temperature increases the solubility of the solute in a solution. In exothermic reactions, increasing the temperature decreases the solubility of the solute.
See some more details on the topic Does temperature increase or decrease solubility in endothermic reactions? here:
Biochemistry, Dissolution and Solubility – StatPearls – NCBI
As temperature increases, the solubility of a solid or liquid can fluctuate depending on whether the dissolution reaction is exothermic or endothermic.
Solubility
Because DHsoln is positive for most solutions, the solution formation reaction is usually endothermic. Therefore, when the temperature is increased, the …
Temperature Changes in Dissolving | Chapter 5 – Middle …
The process of dissolving can be endothermic (temperature goes down) or exothermic (temperature goes up). When water dissolves a substance, the water molecules …
Why does temperature decrease in endothermic reactions?
In an endothermic change, temperature is absorbed from surrounding molecules to continue reacting. If these molecules are losing heat, that means their temperature will drop, resulting in a temperature decrease.
Why a substance with an endothermic heat of solution actually dissolves?
The process of dissolving is endothermic when less energy is released when water molecules “bond” to the solute than is used to pull the solute apart. Because less energy is released than is used, the molecules of the solution move more slowly, making the temperature decrease.
Le Chatelier’s Principle and Temperature Changes (Pt. 10)
Images related to the topicLe Chatelier’s Principle and Temperature Changes (Pt. 10)
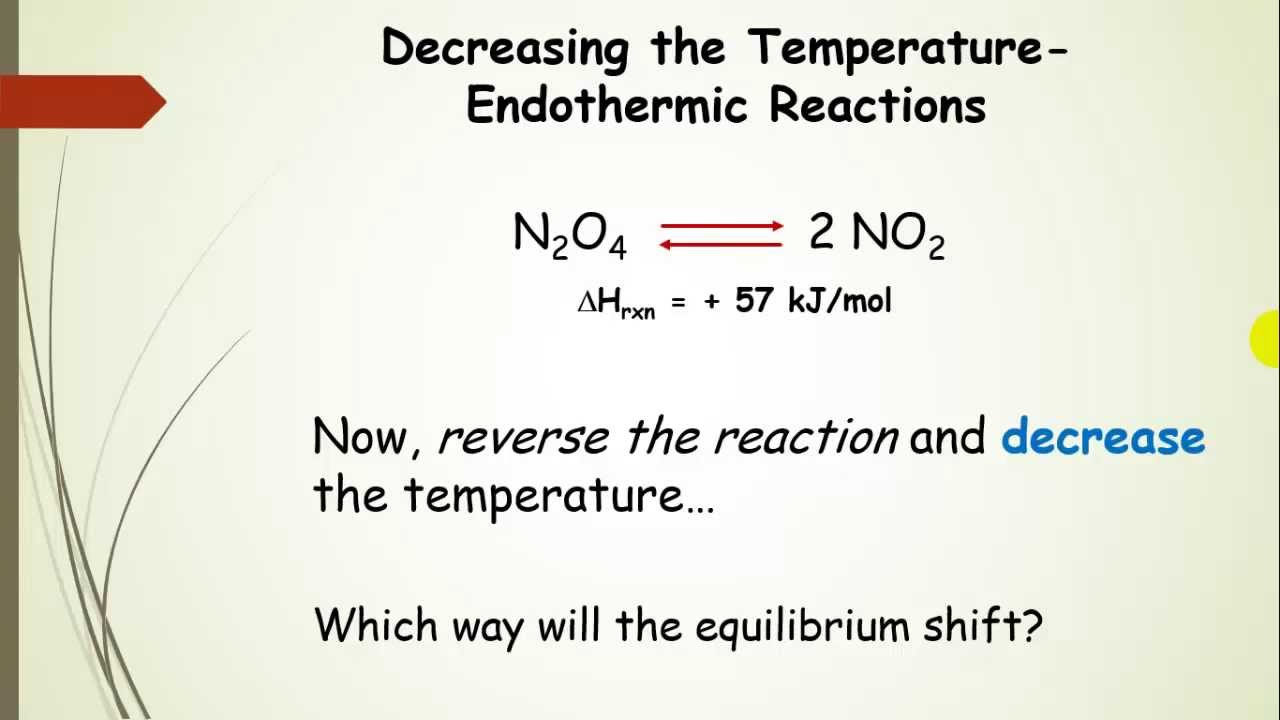
Why does the solubility decrease with temperature?
As the kinetic energy of the gaseous solute increases, its molecules have a greater tendency to escape the attraction of the solvent molecules and return to the gas phase. Therefore, the solubility of a gas decreases as the temperature increases.
Why does an increase in temperature usually increase solubility?
The addition of more heat facilitates the dissolving reaction by providing energy to break bonds in the solid. This is the most common situation where an increase in temperature produces an increase in solubility for solids.
What increases solubility?
An increase in pressure and an increase in temperature in this reaction results in greater solubility. An increase in pressure results in more gas particles entering the liquid in order to decrease the partial pressure. Therefore, the solubility would increase.
Does endothermic increase or decrease temperature?
An exothermic process releases heat, causing the temperature of the immediate surroundings to rise. An endothermic process absorbs heat and cools the surroundings.”
What happens in an endothermic reaction?
An endothermic reaction is any chemical reaction that absorbs heat from its environment. The absorbed energy provides the activation energy for the reaction to occur. A hallmark of this type of reaction is that it feels cold.
What happens when you decrease temperature in an exothermic reaction?
If we approach this problem by thinking about the reaction quotient Q for an exothermic reaction, a decrease in temperature in general causes an increase in the equilibrium constants. And if the equilibrium constant increases, then Q would be less than K. And when Q is less than K, the net reaction goes to the right.
Which temperature had a greater solubility?
If we heat the solvent, the average kinetic energies of its molecules increases. Hence, the solvent is able to dislodge more particles from the surface of the solute. Thus, increasing the temperature increases the solubilities of substances. For example, sugar and salt are more soluble in water at higher temperatures.
Effect of Temperature on Exothermic and Endothermic Reaction|SB SIR | EX FIITJEE FACULTY | IIT BHU
Images related to the topicEffect of Temperature on Exothermic and Endothermic Reaction|SB SIR | EX FIITJEE FACULTY | IIT BHU

What factors affect solubility?
There are two direct factors that affect solubility: temperature and pressure. Temperature affects the solubility of both solids and gases, but pressure only affects the solubility of gases.
What are the 3 factors that affect solubility?
- Temperature: By changing the temperature we can increase the soluble property of a solute. …
- Forces and Bonds: Like dissolves in like. …
- Pressure: Gaseous substances are much influenced than solids and liquids by pressure.
Related searches to Does temperature increase or decrease solubility in endothermic reactions?
- how does temperature affect solubility of gases
- endothermic dissolution
- why does temperature affect solubility
- exothermic dissolution examples
- dissolution chemistry
- the solubility of gases increases with the decrease of
- dissolution vs solubility
- solubility of gas in liquid decreases with the rise in temperature
Information related to the topic Does temperature increase or decrease solubility in endothermic reactions?
Here are the search results of the thread Does temperature increase or decrease solubility in endothermic reactions? from Bing. You can read more if you want.
You have just come across an article on the topic Does temperature increase or decrease solubility in endothermic reactions?. If you found this article useful, please share it. Thank you very much.
