Are you looking for an answer to the topic “Does propanoic acid have hydrogen bonding?“? We answer all your questions at the website Musicbykatie.com in category: Digital Marketing Blogs You Need To Bookmark. You will find the answer right below.
Propanoic acid has hydrogen bonds which are much stronger than the induced-dipole forces in hex-1-ene.Answer and Explanation: The hydrogen bonding exhibited by propanoic acid and water is shown below. It can be observed that both the oxygen atoms as well as the… See full answer below.c) i) The intermolecular forces in propanoic acid will be hydrogen bonds, dipole-dipole forces and London dispersion forces (LDF).

Table of Contents
Does propanoic acid have hydrogen bonding with water?
Answer and Explanation: The hydrogen bonding exhibited by propanoic acid and water is shown below. It can be observed that both the oxygen atoms as well as the… See full answer below.
What type of intermolecular forces are present in propanoic acid?
c) i) The intermolecular forces in propanoic acid will be hydrogen bonds, dipole-dipole forces and London dispersion forces (LDF).
Hydrogen Bonding and Common Mistakes
Images related to the topicHydrogen Bonding and Common Mistakes

What is the main intramolecular force between two molecules of propanoic acid?
The major intermolecular forces between propanoic acid and heptane are dipole-induced dipole forces.
Does propanol have hydrogen bonding?
Molecules of propanol have a hydrogen directly bonded to a NOF atom (oxygen in this case), which means that they can hydrogen bond with eachother. The oxygen in the other three molecules is only bonded to a carbon atom so hydrogen bonding is not possible.
Do carboxylic acids have hydrogen bonding?
In a pure carboxylic acid, hydrogen bonding can occur between two molecules of acid to produce a dimer. This immediately doubles the size of the molecule and so increases the van der Waals dispersion forces between one of these dimers and its neighbors – resulting in a high boiling point.
Is propanoic acid polar?
Both have 3 carbons and both have polar carboxyl groups (-COOH). Also, they both will exhibit hydrogen bonding. But notice that malonic acid has TWO carboxyl groups and this makes it more polar than propanoic acid. Thus, malonic acid should be more soluble in water than propanoic acid.
Does ethanoic acid have hydrogen bonding?
Each ethanoic acid molecule has one O—H bond but is capable of forming double hydrogen bonded dimers as shown in the diagram below. In a pure carboxylic acid two molecules are bonded together using two hydrogen bonds to produce a dimer.
See some more details on the topic Does propanoic acid have hydrogen bonding? here:
Draw the structure of propanoic acid (propionic … – Study.com
The hydrogen bonding exhibited by propanoic acid and water is shown below. It can be observed that both the oxygen atoms as well as the…
Hydrogen bonding in carboxylic acid adlayers on Pd(111)
The adsorption of acetic acid and propanoic acid was studied on the Pd(lll) surface in order togain insights into the extent of hydrogen bonding present in …
15.4 Physical Properties of Carboxylic Acids
Carboxylic acids exhibit strong hydrogen bonding between molecules. They therefore have high boiling points compared to other substances of comparable molar …
21.3: Spectroscopy of Carboxylic Acids – Chemistry LibreTexts
The carboxyl group is associated with two characteristic infrared stretching absorptions which change markedly with hydrogen bonding. The …
What kind of intermolecular force occurs between carboxylic acids?
In a pure carboxylic acid, hydrogen bonding can occur between two molecules of acid to produce a dimer. This immediately doubles the size of the molecule and so increases the van der Waals dispersion forces between one of these dimers and its neighbours – resulting in a high boiling point.
What is the strongest intermolecular force in a carboxylic acid?
…
Acidity.
| Carboxylic Acids | pKa |
|---|---|
| Benzoic acid (C 6H 5CO 2H) | 4.2 |
Is propanoic acid soluble in water?
Carboxylic acids are compounds containing a carboxylic acid group with the formula -C(=O)OH. Thus, propionic acid is considered to be a fatty acid lipid molecule. Propionic acid is soluble (in water) and a weakly acidic compound (based on its pKa).
Which of species can exhibit hydrogen bonding among themselves?
The answer is c. CH3OH C H 3 O H . Hydrogen bonding is a special type of dipole-dipole interaction which only exists when there is a hydrogen…
What are most important intermolecular forces in hydrogen iodide molecules?
In hydrogen iodide are the most important intermolecular forces: dipole-dipole forces covalent bonds polar covalent bonds London dispersion forces hydrogen bonding.
Intermolecular Forces – Hydrogen Bonding, Dipole Dipole Interactions – Boiling Point Solubility
Images related to the topicIntermolecular Forces – Hydrogen Bonding, Dipole Dipole Interactions – Boiling Point Solubility
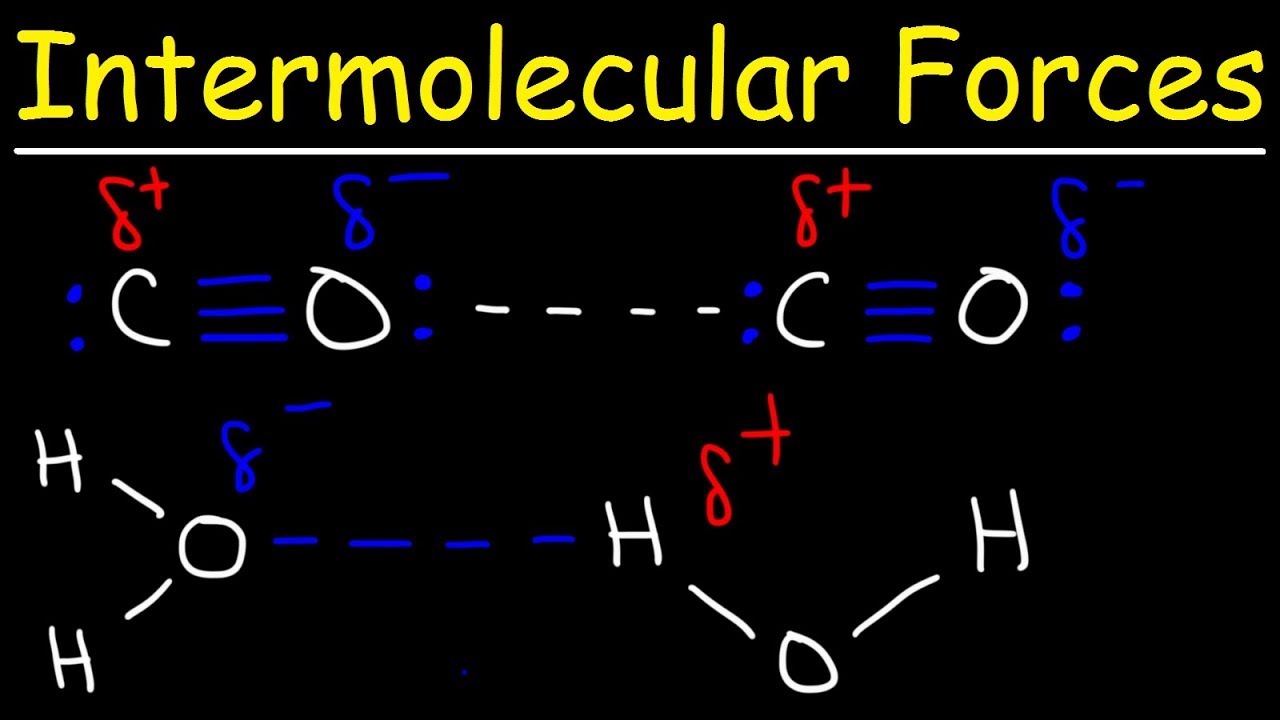
Does propanal show hydrogen bonding?
Abstract. The small alcohols ethanol, 1-propanol, and 2-propanol are miscible in water, form strong hydrogen bonds with water molecules, and are usually known as inhibitors for clathrate hydrate formation.
Can alcohols form hydrogen bonds?
In the case of alcohols, hydrogen bonds occur between the partially-positive hydrogen atoms and lone pairs on oxygen atoms of other molecules. The hydrogen atoms are slightly positive because the bonding electrons are pulled toward the very electronegative oxygen atoms.
What are the intermolecular forces in propanol?
1-Propanol features several different types of intermolecular bonding including London dispersion forces, dipole-dipole interactions, and hydrogen bonding. Of these, the hydrogen bonds are known to be the strongest.
How many hydrogen bonds can a carboxylic acid form?
The difference is that two molecules of a carboxylic acid form two hydrogen bonds with each other (two alcohol molecules can only form one). Thus, carboxylic acids exist as dimers (pairs of molecules), not only in the liquid state but even to some extent in the gaseous state.
Why do carboxylic acids have stronger hydrogen bonding than alcohols because?
The hydrogen bond formed by the carboxylic acids are stronger than those in alcohols because O−H bond in COOH is more strongly polarised due to the presence of electron withdrawing carboxy group in adjacent position then the O−H bonds of alcohols.
What is carboxylic hydrogen?
Carboxylic acids are polar. Because they are both hydrogen-bond acceptors (the carbonyl –C=O) and hydrogen-bond donors (the hydroxyl –OH), they also participate in hydrogen bonding. Together, the hydroxyl and carbonyl group form the functional group carboxyl.
Is propanoic acid ionic or covalent?
Salts of carboxylic acids
Propanoic acid is a three carbon acid with no carbon-carbon double bonds. Notice that there is an ionic bond between the sodium and the propanoate group.
What type of compound is propanoic acid?
Propionic acid (/proʊpiˈɒnɪk/, from the Greek words protos, meaning “first”, and pion, meaning “fat”; also known as propanoic acid) is a naturally occurring carboxylic acid with chemical formula CH3CH2CO2H. It is a liquid with a pungent and unpleasant smell somewhat resembling body odor.
Why propanoic acid is soluble in water?
In propanoic acid, there is only one alkanoic group(COOH) that make dipole dipole attraction with water for the purpose of solubility, While in propandioic acid there are two alkanoic groups(COOH) that makes bonds with water for solubility, That’s why propandioic acid is more soluble in water than propanoic acid.
Which compound has hydrogen bonding between its molecules?
…
| element | electronegativity value |
|---|---|
| H | 2.1 |
| N | 3.0 |
| O | 3.5 |
| F | 4.1 |
Hydrogen Bonds – What Are Hydrogen Bonds – How Do Hydrogen Bonds Form
Images related to the topicHydrogen Bonds – What Are Hydrogen Bonds – How Do Hydrogen Bonds Form

Is CH3 3N hydrogen bonding?
In (CH3)3N ( C H 3 ) 3 N , the hydrogen atoms are bonded to carbon atoms. Carbon is not a very electronegative atom so it cannot act as a hydrogen donor. Although nitrogen is very electronegative and can act as a hydrogen acceptor, there are no hydrogens to accept.
Does HCl have hydrogen bonding?
The size of the atom, considering its electronegativity, is such that its electron density is too low for hydrogen bonds to form. This is why, while HF does, HCl does not demonstrate hydrogen bonding.
Related searches to Does propanoic acid have hydrogen bonding?
- does propanoic acid have hydrogen bonding
- does ethanoic acid have hydrogen bonding
- does acetic acid have hydrogen bonding
- intermolecular forces in butanoic acid
- does propanoic acid form hydrogen bonds with water
- intermolecular forces in propanol
- why does ethanoic acid have a higher boiling point than ethanol
- what causes hydrogen bonding
- does nitric acid have hydrogen bonding
- does sulfuric acid have hydrogen bonding
- intermolecular forces in ethanoic acid
- can propanoic acid form hydrogen bonds
- what is acidic bonding
- why does ethanoic acid have a high boiling point
- does h2so4 have hydrogen bonding
- why do carboxylic acids have higher boiling points than alcohols
- does octane have hydrogen bonding
Information related to the topic Does propanoic acid have hydrogen bonding?
Here are the search results of the thread Does propanoic acid have hydrogen bonding? from Bing. You can read more if you want.
You have just come across an article on the topic Does propanoic acid have hydrogen bonding?. If you found this article useful, please share it. Thank you very much.
