Are you looking for an answer to the topic “Does potassium form a covalent bond?“? We answer all your questions at the website Musicbykatie.com in category: Digital Marketing Blogs You Need To Bookmark. You will find the answer right below.
Potassium hydroxide, KOH, contains one bond that is covalent (O-H) and one that is ionic (K-O). Hydrogen is tricky because it is at the top of the periodic table as well as the left side. It is just electropositive enough to form
in some cases.Explanation: Potassium iodide (KI) forms an ionic bond. Potassium and iodine have very different electronegativities. The two atoms would form an ionic bond since ionic bonds form between atoms with a large difference in electronegativity (difference>1.7 using the Pauling scale will result in an ionic bond).Explanation: As potassium is a metal, metallic bond is present in solid potassium.

Does potassium form ionic or covalent bonds?
Explanation: Potassium iodide (KI) forms an ionic bond. Potassium and iodine have very different electronegativities. The two atoms would form an ionic bond since ionic bonds form between atoms with a large difference in electronegativity (difference>1.7 using the Pauling scale will result in an ionic bond).
What type of bonds do potassium form?
Explanation: As potassium is a metal, metallic bond is present in solid potassium.
Introduction to Ionic Bonding and Covalent Bonding
Images related to the topicIntroduction to Ionic Bonding and Covalent Bonding

Is potassium chloride ionic or covalent?
The type of chemical bond that holds together the potassium and chlorine atoms in a potassium chloride molecule is an ionic bond.
Is potassium and calcium ionic or covalent?
No for a couple of reasons: Ionic bonds are between a metal and a non-metal – Ca and K are both metals. K and Ca have a difference of . 18 in the Pauling Scale – and definitions of ionic bonds are often stated as having above a 1.7 difference.
How do you know if it is ionic or covalent bond?
By definition, an ionic bond is between a metal and a nonmetal, and a covalent bond is between 2 nonmetals. So you usually just look at the periodic table and determine whether your compound is made of a metal/nonmetal or is just 2 nonmetals.
Why does KCl not form covalent bonds?
Potassium (K) has atomic number 19 and chlorine (Cl ) has atomic number 17. So, there is deficiency of one electron in Cl atom to complete its octet. Thus, K will transfer its one electron to the Cl atom. Thus, KCl will be an ionic compound as the bond formation is due to the transfer of electron.
How do you know if a compound is covalent?
- If a compound is made from a metal and a non-metal, its bonding will be ionic.
- If a compound is made from two non-metals, its bonding will be covalent.
See some more details on the topic Does potassium form a covalent bond? here:
Covalent Compounds – University of Hawaii at Manoa
In some cases, three covalent bonds can be formed between two atoms. … two K+ ions to balance the charge of one (SiO2)2- ion to form potassium silicate.
Is potassium likely to form a covalent bond? – AnswersToAll
When the two atoms are in contact, potassium readily transfers its outer electron to chlorine which readily accepts it, resulting in both atoms …
MCAT Physical : Compounds, Molecules, and Bonds – Varsity …
Potassium iodide (KI) forms an ionic bond. Potassium and iodine have very different electronegativities. The two atoms would form an ionic bond since ionic …
Is KCl Covalent or Ionic? – Techiescientist
Therefore, Potassium and Chlorine being oppositely charged ions attract each other and due to large electronegativity difference forms a chemical bond resulting …
Is potassium iodide covalent or ionic?
Potassium iodide is a metal-halide salt featuring an ionic bond between the potassium cation (K+) and the iodide anion (I–).
Is potassium bromide ionic or covalent?
Potassium bromide has ionic bonding between its two elements potassium and bromine.
Is sodium ionic or covalent?
…
Table 2.11.
| Property | Ionic | Covalent |
|---|---|---|
| Melting temperature | High | Low |
What type of solid is potassium?
Classified as an alkali metal, Potassium is a solid at room temperature.
Ionic and Covalent Bonding – Chemistry
Images related to the topicIonic and Covalent Bonding – Chemistry
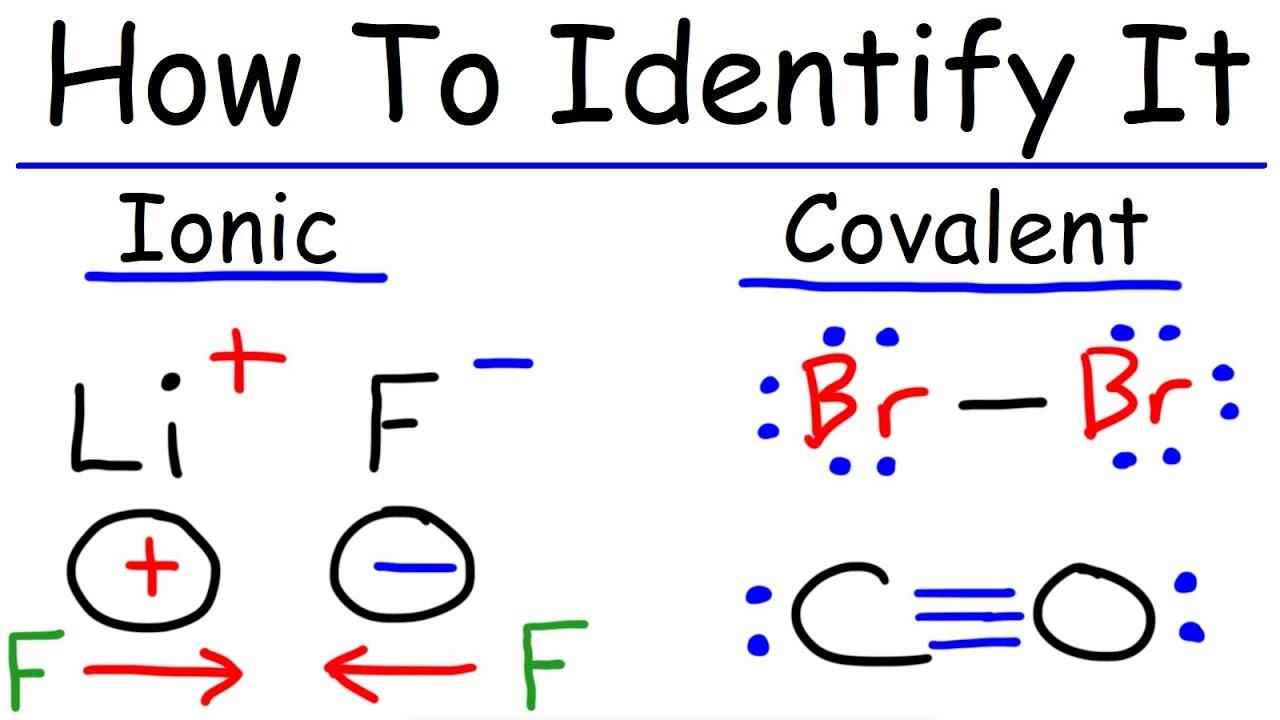
Is potassium and chlorine a covalent bond?
So, is KCl ionic or covalent? Yes, KCl is ionic in nature as the electronegativity of Potassium is 0.82 while that of Chlorine is 3.16 and hence, the difference between the electronegativity of these two elements is 2.34 which is greater than 2.0, required for the formation of ionic bond.
What type of bond is potassium and chloride?
Due to the formation of ionic bond between potassium and chlorine atom, potassium chloride is formed. The bond formation is ionic in nature since the electron is transferred between the two atoms.
What kind of elements form covalent bonds?
What elements make covalent bonds? Covalent bonds form when two or more nonmetals combine. For example, both hydrogen and oxygen are nonmetals, and when they combine to make water, they do so by forming covalent bonds.
What type of bond is potassium and calcium?
…
Naming and Formulas for Ionic Compounds.
| Calcium Fluoride = | Potassium Chloride = |
|---|---|
| Lithium Oxide = | Aluminum Sulfide = |
Which elements form both ionic and covalent bonds?
Calcium carbonate is another example of a compound with both ionic and covalent bonds. Here calcium acts as the cation, with the carbonate species as the anion. These species share an ionic bond, while the carbon and oxygen atoms in carbonate are covalently bonded.
Is potassium oxide a covalent compound?
Potassium oxide is an ionic compound formed by combining potassium and oxygen.
What is covalent bond and examples?
By definition, a pure covalent bond is one that exists between two atoms with the same electronegativities. Thus, a pure covalent bond does not display any ionic character. Diatomic elements are perfect examples of pure covalent bonds because both the atoms evenly share the electrons. Examples: H2, O2, and N2.
What two types of atoms make a covalent bond?
Covalent bonds form between atoms of nonmetallic elements. In general, bonds are considered to be covalent if the electronegativity difference between the two atoms bonding is less than 2.0 Pauling units.
Does KCl contain both ionic and covalent bonds?
Answer and Explanation: Potassium chloride, KCl, is an ionic salt in which potassium donates an electron to chlorine creating two charged ions drawn together by opposite… See full answer below.
This is how the ionic bond forms in Potassium Chloride (KCl).
Images related to the topicThis is how the ionic bond forms in Potassium Chloride (KCl).

Which of the following has no covalent bond?
Answer: (2)
Compound having no covalent bonds is KCl only.
Which of the following does not have covalent bond?
So CoCl2 is an ionic compound and doesn’t have covalent bonds.
Related searches to Does potassium form a covalent bond?
- what type of elements form covalent bonds
- hcl ionic or covalent
- is sodium chloride ionic or covalent
- how to tell if a bond is ionic or covalent using electronegativity
- does potassium and oxygen form a covalent bond
- is potassium chloride ionic or covalent
- does potassium and phosphorus form a covalent bond
- what type of bond does potassium form
- can k and f form a covalent bond
- how many covalent bonds can indium form
- does potassium form covalent bonds
- does potassium and calcium form a covalent bond
- how many bonds can potassium form
- how is covalent bond form
- can potassium and chlorine form a covalent bond
- is potassium iodide ionic or covalent
- does potassium and chlorine form a covalent bond
- what happens when a covalent bond forms
Information related to the topic Does potassium form a covalent bond?
Here are the search results of the thread Does potassium form a covalent bond? from Bing. You can read more if you want.
You have just come across an article on the topic Does potassium form a covalent bond?. If you found this article useful, please share it. Thank you very much.
