Are you looking for an answer to the topic “Does internal energy only depend on temperature?“? We answer all your questions at the website Musicbykatie.com in category: Digital Marketing Blogs You Need To Bookmark. You will find the answer right below.
The internal energy and enthalpy of
depends only on temperature, not on volume or pressure.In an ideal gas the inter-molecular collisions are assumed to be absent and the collisions are perfectly elastic. Thus, the gas possesses only translational kinetic energy and hence the internal energy of the ideal gas depends only on temperature.Doing work is transferring energy. So you transferred energy to the sticks and their temperature increased. This means that an increase in the temperature of a system is an indication of an increase in the internal energy (a.k.a. thermal energy) of the system.

Why internal energy of an real gas depends only on temperature?
In an ideal gas the inter-molecular collisions are assumed to be absent and the collisions are perfectly elastic. Thus, the gas possesses only translational kinetic energy and hence the internal energy of the ideal gas depends only on temperature.
How is internal energy related to temperature?
Doing work is transferring energy. So you transferred energy to the sticks and their temperature increased. This means that an increase in the temperature of a system is an indication of an increase in the internal energy (a.k.a. thermal energy) of the system.
Dependence of Internal Energy on Temperature
Images related to the topicDependence of Internal Energy on Temperature

Does the internal energy of a gas depends only on the gas temperature?
The internal energy of an ideal gas depends only on its temperature, not on its pressure or volume. So, obviously, the internal energy (U) depends only on the temperature (T) and the number of moles (n) of the gas.
Why is internal energy only a function of temperature?
Pressure and volume change while the temperature remains constant. Since no work or heat are exchanged with the surrounding, the internal energy will not change during this process. Thus, the internal energy of an ideal gas is only a function of its temperature.
On which of the following factors internal energy depends?
Hence, the internal energy of a fixed mass of gas depends on its temperature and its density (or, which is the same for a given temperature, its volume).
Is internal energy the same as temperature?
Temperature is related to the kinetic energies of the molecules of a material. It is the average kinetic energy of individual molecules. Internal energy refers to the total energy of all the molecules within the object.
How does internal energy of a substance vary with temperature?
The internal energy of a substance increases with increase in temperature due to increase in rotational, translational and vibrational energy of the molecule.
See some more details on the topic Does internal energy only depend on temperature? here:
INTERNAL ENERGY – Thermopedia
Pressure and volume change while the temperature remains constant. Since no work or heat are exchanged with the surrounding, the internal energy will not change …
Internal energy – Wikipedia
Thus, its value depends only on the current state of the system and not on the particular choice from many possible processes by which energy may pass to or …
Energy, Enthalpy, and the First Law of Thermodynamics
Because the internal energy of the system is proportional to its temperature, internal energy is also a state function. Any change in the …
why does internal energy depend only on temperature – Physics
Internal energy depends on following factors: Temperature: If the temperature of a system rises, the molecules will travel quicker, therefore …
What happens to internal energy when temperature decreases?
Now, Q here is heat inside system which is a measure of Temperature and not energy of system. Temperature decreases and hence Q decreases. Since ΔT decreases but n moles inside systems increase which compensate more than decrease in Temperature and hence internal energy of system increases.
Internal Energy
Images related to the topicInternal Energy
Why does kinetic energy only depend on temperature?
Anything that changes the temperature means that the average kinetic energy per molecule has changed. This is what being dependent only on temperature means. The ideal gas equation of state is PV=nRT … so you see that temperature is related to the other state variables too.
Why is the internal energy of a gas only kinetic energy?
Matterwave said: In an ideal gas (that we regularly study), there is assumed to be no interactions between the gas molecules. No interactions means no potential energy, so the only form of energy left is kinetic.
Which law states that the internal energy of a perfect gas depends only on its temperature?
Joule’s law: This law states that the internal energy of a given quantity of a gas depends only on the temperature. It is independent of pressure and volume.
Is internal energy a function of temperature and volume?
The internal energy of a real gas is function of both, temperature and volume.
Why internal energy does not depend on pressure?
Internal energy and enthalpy of ideal gases depend only on temperature. Previously, we said that the enthalpy of an ideal gas is independent of pressure at constant temperature. And the internal energy of an ideal gas is independent of volume at constant temperature.
What is internal energy explain it?
internal energy, in thermodynamics, the property or state function that defines the energy of a substance in the absence of effects due to capillarity and external electric, magnetic, and other fields.
U depends on T only
Images related to the topicU depends on T only
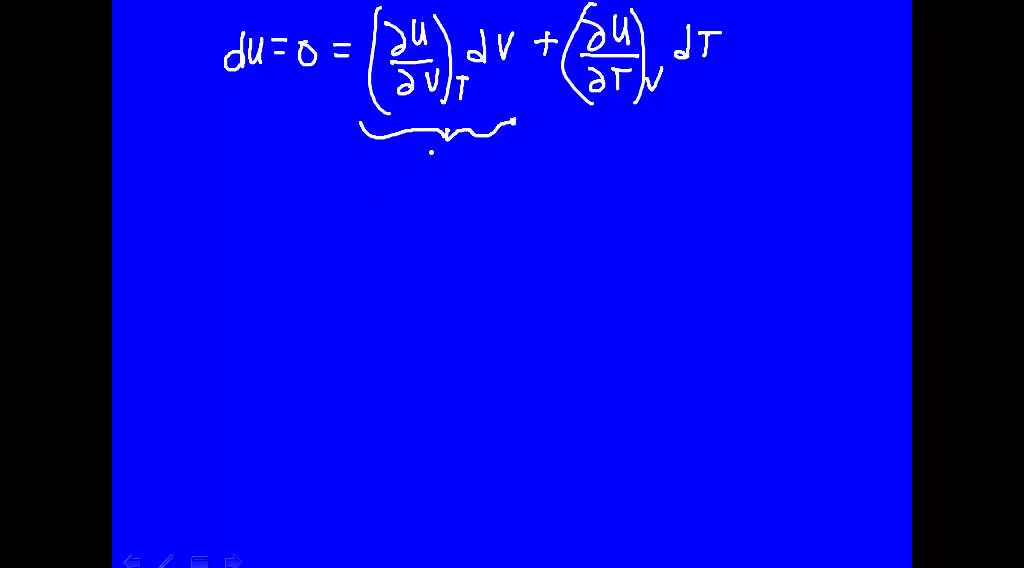
What does internal energy of an ideal gas depend on?
The internal energy and enthalpy of ideal gases depends only on temperature, not on volume or pressure.
Is internal energy a function of pressure?
Answer : The internal energy of an ideal gas dependes on the temperature of the gas only while that of a real gas depends on the temperature and pressure. Thus, the internal energy of an ideal gas is not a function of its pressure but that of a real gas is a function of pressure also.
Related searches to Does internal energy only depend on temperature?
- the enthalpy and internal energy are the function of temperature for
- internal energy for real gases
- does internal energy depends on temperature
- why internal energy depends on temperature
- internal energy of an ideal gas
- how to find internal energy with pressure and temperature
- why is internal energy only dependent on temperature
- why does the internal energy of an ideal gas only depends on temperature
- why does internal energy only depends on temperature
- what happens to internal energy when temperature increases
- internal energy of an ideal gas formula
- internal energy of a perfect gas depends on
Information related to the topic Does internal energy only depend on temperature?
Here are the search results of the thread Does internal energy only depend on temperature? from Bing. You can read more if you want.
You have just come across an article on the topic Does internal energy only depend on temperature?. If you found this article useful, please share it. Thank you very much.
