Are you looking for an answer to the topic “Do all electrons ejected from the metal surface have the same kinetic energy?“? We answer all your questions at the website Musicbykatie.com in category: Digital Marketing Blogs You Need To Bookmark. You will find the answer right below.
Thus, each electron will have a slightly different kinetic energy after it gets ejected from the metal.Answer: No. Each electron energy possessed by an ejected electron can either be the same or different from the other because when a photon energy is absorbed by an electron, part of the energy is used to overcome the binding energy. The extra energy is the one used to release the electron.In process of photoelectric emission, all emitted electrons do not have same kinetic energy.

Table of Contents
Do all emitted electrons have the same kinetic energy?
Answer: No. Each electron energy possessed by an ejected electron can either be the same or different from the other because when a photon energy is absorbed by an electron, part of the energy is used to overcome the binding energy. The extra energy is the one used to release the electron.
Do emitted photoelectrons have the same kinetic energy?
In process of photoelectric emission, all emitted electrons do not have same kinetic energy.
Photoelectric Effect – Will electrons be ejected from the metal? (From Wavelength)
Images related to the topicPhotoelectric Effect – Will electrons be ejected from the metal? (From Wavelength)

Is kinetic energy of all photoelectrons from a given metal is same?
1 Answer. No. Depending upon the position and state of an electron in a metal when it absorbs an incident photon, a photoelectron can have kinetic energy ranging from 0 to a certain maximum value equal to the photon energy minus the work function of the metal.
Why photoelectrons ejected from a metal surface have different kinetic energies?
The electrons in the atom of metal occupy different energy levels, thus have different minimum energy required to be ‘ejected’ from the atom. So the `e^-` with higher energy will have higher kinetic energy.
Why do emitted electrons have a range of kinetic energies?
electron loses various amounts of energy in order to reach the surface of metal. electron transfers ‘work function’ energy to escape from the surface. electrons have a maximum kinetic energy = (photon energy) − work function.
Why all the photo electrons do not have the same energy?
All the electrons in an atom do not have the same energy level. When a ray having some photon energy is incident on a metal surface, the electrons come out from different levels with different energies. Hence, these emitted electrons show different energy distributions.
On what factors does the number of emitted photoelectrons depend?
The number of photoelectrons depend on the number of photons emitted when an electromagnetic radiation hits the material. That is no of photons incident per second is termed as intensity. Hence, the number of photoelectrons emitted depends on the intensity of the incident radiation.
See some more details on the topic Do all electrons ejected from the metal surface have the same kinetic energy? here:
Is the kinetic energy of all photoelectrons the same … – Quora
No. Each electron energy possessed by an ejected electron can either be the same or different from the other because when a photon energy is absorbed by an …
Solved Do all electrons ejected from the metal surface have
Question: Do all electrons ejected from the metal surface have the same kinetic energy? Is this important for the interpretation of this lab?
Do all electrons ejected from a metal surface have … – Study.com
No, it is not necessary that all the ejected electrons will have the same energy. Because the kinetic energy of the electron is the excess energy than.
Photoelectric effect (article) | Photons | Khan Academy
For frequencies greater than ν 0 \nu_0 ν0\nu, start subscript, 0, end subscript, electrons would be ejected from the metal. Furthermore, the kinetic energy of …
What is the relation between kinetic energy and frequency of the photoelectrons?
1 Answer. Kinetic energy of the ejected electron is proportional to the frequency of the electromagnetic radiation.
When light is incident on surface photoelectrons are emitted For photoelectrons?
A photosensitive surface ejects electrons when green light is incident on it. It does not emit any photoelectrons when exposed to orange light.
Photoelectric Effect, Work Function, Threshold Frequency, Wavelength, Speed Kinetic Energy, Electr
Images related to the topicPhotoelectric Effect, Work Function, Threshold Frequency, Wavelength, Speed Kinetic Energy, Electr
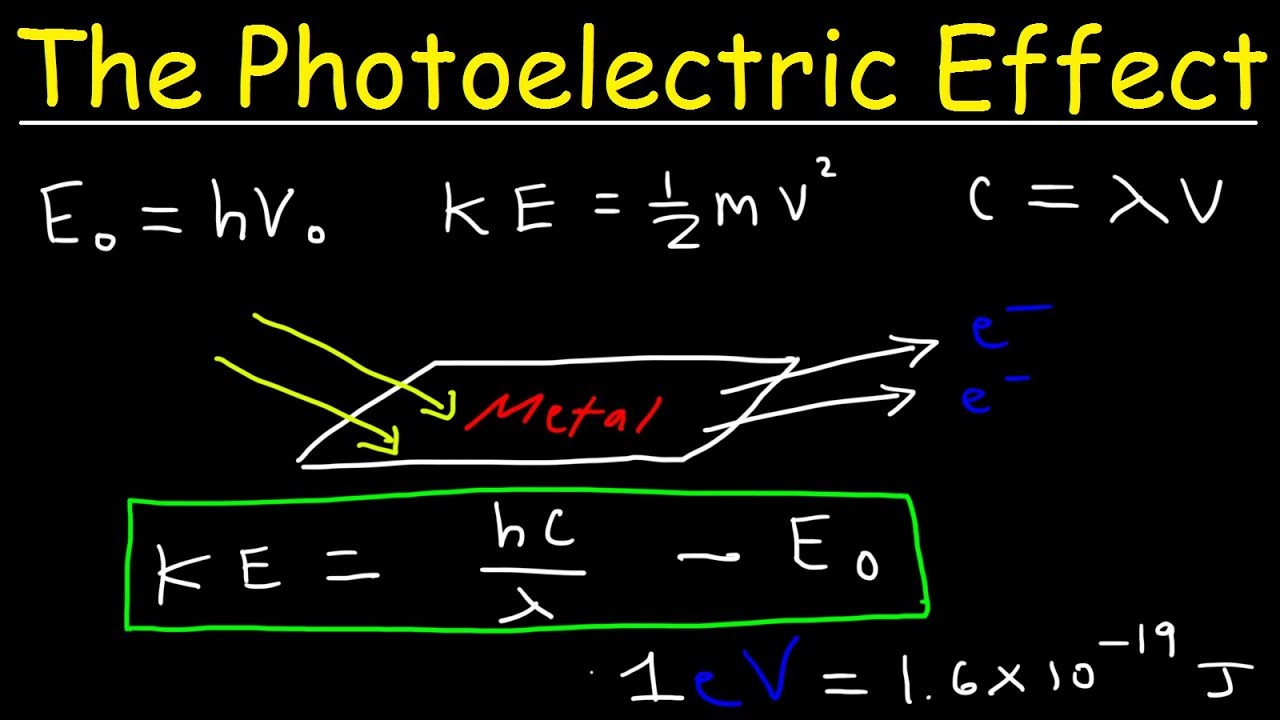
Why photoelectric emission is not possible for all frequencies?
Photoelectric emission is not possible at all frequencies because for the emission to occur there should be a minimum value of frequency related to the work function of the metal.
Why the photoelectrons have a different range of kinetic energy and how it is related to the frequency of the incident light?
The kinetic energy of photoelectrons increases with light frequency. Electric current remains constant as light frequency increases. Electric current increases with light amplitude. The kinetic energy of photoelectrons remains constant as light amplitude increases.
Why does the kinetic energy of photoelectrons vary up to a maximum value?
I think its because when the light hits the surface, photons of energy are given to the electrons, and the energy of the photons vary, so not every electron would get hit by photons of the same energy levels, giving them a different amount of kinetic energy.
How do you find the kinetic energy of a photoelectron?
The maximum kinetic energy of a photoelectron is given by 𝐸 = ℎ 𝑐 𝜆 − 𝑊 , m a x where ℎ is the Planck constant, 𝑐 is the speed of light, 𝜆 is the wavelength of the incident photon, and 𝑊 is the work function of the metal surface.
Why do photoelectrons all have the same energy when illuminated with a given frequency of light?
Why do photoelectrons all have the same energy when illuminated with a given frequency of light? Each photon has the same energy, which gets transferred to a single photoelectron.
Why photoelectric effect does not occur for free electrons?
A photoelectric effect is the absorption of a gamma quantum by an electron, in which the gamma quantum gives up almost its entire energy to the electron. Absorption by a free electron is impossible, as the law of momentum conservation is not satisfied.
When a clean metal surface in a vacuum is irradiated?
When a clean metal surface in a vacuum is irradiated with ultraviolet radiation of a certain frequency, electrons are emitted from the metal.
Why do all photo electrons not come out with same energy of incident radiation is monochromatic?
Since electrons are present in a continuous band of levels, different electrons require different amounts of energy to get out of the atom. For this reason electrons knocked off by a monochromatic radiation posses different energies.
Will electrons be ejected from the metal? (Photoelectric Effect Example)
Images related to the topicWill electrons be ejected from the metal? (Photoelectric Effect Example)

Why do all electrons emitted in beta decay do not have the same energy?
The available energy is shared by electrons and antineutrinos in all proportions. That is why all electrons emitted during β−decay not have the same energy.
Which factor determine the maximum kinetic energy of an emitted electron?
The distribution of energies of emitted electrons doesn’t depend on the intensity of the light. Whether electrons are emitted at all depends on the frequency of the light being high enough. The maximum kinetic energy depends only on the frequency of the light.
Related searches to Do all electrons ejected from the metal surface have the same kinetic energy?
- work function formula
- e hf w
- kinetic energy of electron
- photoelectric effect physics
- photoelectric effect equation
- do all electrons ejected from the metal surface have the same kinetic energy
- e = hf – w
- work function photoelectric effect
Information related to the topic Do all electrons ejected from the metal surface have the same kinetic energy?
Here are the search results of the thread Do all electrons ejected from the metal surface have the same kinetic energy? from Bing. You can read more if you want.
You have just come across an article on the topic Do all electrons ejected from the metal surface have the same kinetic energy?. If you found this article useful, please share it. Thank you very much.
