Table of Contents
What is the difference between Markovnikov rule and anti-Markovnikov rule?
Markovnikov’s rule is like a rule of thumb that tells us where the hydrogen atom from the hydrogen halide will attach to an alkene. It states that the hydrogen will bond to the carbon with the most hydrogen atoms already attached. Think of it as the hydrogen trying to “hang out” with the carbon that’s already got the most friends.
Anti-Markovnikov’s rule is the opposite. In this case, the hydrogen attaches to the carbon with the fewest hydrogen atoms already attached. It’s like the hydrogen trying to be a bit of a rebel and go against the crowd.
Now, why are these rules important?
Markovnikov’s rule is key for making alkyl halides, which are organic compounds containing a halogen atom bonded to a carbon atom. They’re often used in making plastics, pesticides, and other useful substances.
Anti-Markovnikov’s rule is the go-to for synthesizing alcohols, which are organic compounds that contain a hydroxyl group (OH). They’re crucial in everything from drinks to pharmaceuticals.
Think of it like this:
Imagine you have a group of friends, and you’re trying to decide where to go for dinner.
* If you follow Markovnikov’s rule, you’d go to the restaurant that has the most people you know already.
* If you follow anti-Markovnikov’s rule, you’d go to the restaurant where you know the fewest people, perhaps for a change of pace.
Both rules have their own unique applications in chemistry, and understanding them is essential for anyone studying organic chemistry.
How to know Markovnikov vs Anti-Markovnikov?
The key difference lies in where the hydrogen and nucleophile attach to the alkene. In a Markovnikov reaction, the hydrogen goes to the carbon with more hydrogens, while the nucleophile goes to the carbon with fewer hydrogens. Think of it as the hydrogen going to the “richer” carbon.
On the other hand, Anti-Markovnikov reactions are the opposite. The hydrogen attaches to the carbon with fewer hydrogens, and the nucleophile attaches to the carbon with more hydrogens. In essence, the hydrogen goes to the “poorer” carbon.
So, how can you tell them apart? It’s all about the reagent used. Markovnikov reactions usually involve hydrohalogenation using HX (like HCl, HBr, or HI), while Anti-Markovnikov reactions often involve hydroboration-oxidation using BH3.
The hydroboration-oxidation process involves two steps:
1. Hydroboration: BH3 adds to the alkene, forming an alkylborane. BH3 is a Lewis acid, so it attacks the alkene and forms a bond with the less substituted carbon.
2. Oxidation: The alkylborane is then oxidized by hydrogen peroxide (H2O2) and sodium hydroxide (NaOH) to produce an alcohol. The oxidation step replaces the boron with a hydroxyl group.
Remember, hydroboration-oxidation follows Anti-Markovnikov regiochemistry. This is because the boron atom in BH3 is more electronegative than hydrogen, making the boron a partial positive and the hydrogen a partial negative. The boron then attacks the less substituted carbon, resulting in the Anti-Markovnikov product.
What is the difference between Markovnikov and peroxide effect?
You’re right, the peroxide effect is the opposite of Markovnikov’s rule. Markovnikov’s rule tells us that when a hydrogen halide (like HCl or HBr) adds to an alkene, the hydrogen atom will attach to the carbon atom that already has more hydrogen atoms.
Think of it this way: hydrogen prefers to hang out with other hydrogens!
On the other hand, the peroxide effect flips this behavior on its head. When we introduce a peroxide (like hydrogen peroxide or benzoyl peroxide) as a catalyst, the hydrogen atom in the hydrogen halide will now attach to the carbon with fewer hydrogen atoms.
This change in behavior is due to the free radical mechanism that happens when peroxides are present. Peroxides break down into free radicals, which are very reactive and influence the way the hydrogen halide adds to the alkene.
Let’s look at a simple example. Imagine you have propene (a three-carbon alkene) reacting with HBr. Without a peroxide, the Markovnikov product would be 2-bromopropane, as the hydrogen adds to the carbon with two hydrogens, and the bromine attaches to the carbon with one hydrogen.
But, if we add peroxide to the mix, the peroxide effect takes over! The hydrogen from HBr will now attach to the carbon with only one hydrogen, leading to 1-bromopropane.
It’s like the peroxide says, “Hey, hydrogen, let’s mix things up and go against the usual rules!”
So, in a nutshell, Markovnikov’s rule and the peroxide effect describe two different ways that hydrogen halides can add to alkenes, and they all come down to how the hydrogen atom decides where to attach.
Why do we use anti Markovnikov rule?
But, anti-Markovnikov reactions are special. They happen because a special type of molecule called a radical is involved. Radicals are atoms or molecules with an unpaired electron, making them very reactive. In anti-Markovnikov reactions, the radical attacks the alkene or alkyne, leading to the formation of a new carbon radical. This carbon radical then reacts with another molecule, and the final product ends up with the substituent on the less substituted carbon.
Let’s break down how this works with an example. Imagine you have an alkene reacting with hydrogen bromide (HBr). In a typical Markovnikov reaction, the hydrogen (H) atom would add to the more substituted carbon, while the bromine (Br) atom would add to the less substituted carbon.
However, if the reaction is done in the presence of a radical initiator, like peroxides, the reaction takes a different path. The radical initiator generates a bromine radical (Br•). This bromine radical then attacks the alkene, forming a carbon radical. The carbon radical is more stable on the less substituted carbon, so the bromine (Br) atom ends up attaching there.
This anti-Markovnikov addition is a very useful reaction in organic chemistry. It allows us to create molecules with specific structures and properties. So, while it might seem counterintuitive at first, understanding the role of radicals helps us understand why anti-Markovnikov reactions happen and how they can be used to our advantage.
Is anti Markovnikov more stable?
Anti-Markovnikov addition is a reaction where a reagent, like HBr, adds to an alkene in a way that’s opposite to what you’d expect based on Markovnikov’s rule. Markovnikov’s rule states that in the addition of a protic acid to an alkene, the hydrogen atom will bond to the carbon atom with the most hydrogen atoms already attached.
Why does the anti-Markovnikov addition happen?
It’s all about the presence of radicals or heat. These conditions can initiate a free-radical reaction mechanism.
Let’s look at an example: The reaction of HBr with an alkene. In a typical reaction, the hydrogen atom in HBr would add to the carbon with more hydrogens, following Markovnikov’s rule.
However, in the presence of heat or peroxide, the HBr undergoes homolytic cleavage. This means the H-Br bond breaks, forming a hydrogen radical and a bromine radical.
The bromine radical is much more stable than the hydrogen radical. So, when these radicals react with the alkene, the bromine radical preferentially adds to the carbon with fewer hydrogen atoms. This is because the resulting carbon radical will be more stable, due to the presence of more alkyl groups.
Stability is key!
The anti-Markovnikov product, formed by the bromine radical adding first, ultimately leads to a more stable carbocation. This carbocation then reacts with the hydrogen atom, resulting in the anti-Markovnikov product.
So, while the anti-Markovnikov product might not be the most stable initially, it’s the most stable product you get after going through a series of radical reactions. This is why heat or peroxide are essential for this reaction to happen.
Let’s recap:
Anti-Markovnikov addition occurs in the presence of radical initiators or heat.
These conditions lead to the formation of free radicals.
The more stable radical (bromine radical in the case of HBr) adds to the less substituted carbon.
This results in a more stable intermediate carbocation, leading to the anti-Markovnikov product.
I hope this helps clear up any confusion around the stability of the anti-Markovnikov product!
Is Markovnikov rule valid?
Markovnikov’s rule is a fundamental concept in organic chemistry that helps us predict the product of an electrophilic addition reaction to an alkene. It states that the hydrogen atom of the hydrogen halide (like HCl or HBr) will add to the carbon atom with the most hydrogen atoms already attached, while the halogen atom will attach to the carbon atom with fewer hydrogen atoms. This is because the carbocation intermediate formed during the reaction is more stable when it has more alkyl groups attached to it, which helps to disperse the positive charge.
So, is Markovnikov’s rule always valid? The answer is not quite. While it works for a large number of reactions, there are certain scenarios where it needs to be considered with caution:
Presence of Peroxides: When peroxides are present, the reaction undergoes a radical mechanism instead of the typical electrophilic addition. This leads to an anti-Markovnikov addition product, where the hydrogen atom adds to the carbon with fewer hydrogen atoms, and the halogen atom adds to the carbon with more hydrogen atoms.
Carbocation Rearrangements: Sometimes, a more stable carbocation intermediate can be formed through a 1,2-hydride shift or a 1,2-alkyl shift. In such cases, the final product will not follow the original Markovnikov’s rule prediction.
The core principle of Markovnikov’s rule remains true: the reaction will favor the most stable carbocation intermediate. However, the most stable carbocation might not always be the one formed directly after the initial proton addition. We need to consider possible rearrangements that might occur to achieve the most stable carbocation, and that’s where Markovnikov’s rule needs to be applied carefully.
Here’s an example to illustrate:
Imagine you are adding HBr to propene. According to Markovnikov’s rule, the hydrogen atom would attach to the terminal carbon, and the bromine atom would attach to the middle carbon, forming 2-bromopropane. This is because the secondary carbocation intermediate formed by attaching bromine to the middle carbon is more stable than the primary carbocation that would form if bromine attached to the terminal carbon.
But, if you add a peroxide to the reaction mixture, the reaction follows a radical mechanism, and you get 1-bromopropane instead. This is because the radical intermediate formed by attaching bromine to the terminal carbon is more stable than the radical intermediate formed by attaching bromine to the middle carbon.
The key takeaway:
Markovnikov’s rule is a valuable tool for predicting the outcome of many electrophilic additions.
* However, it’s important to remember that it’s not an absolute rule.
* You should always consider the possibility of carbocation rearrangements and the presence of peroxides when applying Markovnikov’s rule.
Where does Markovnikov rule apply?
Let’s break it down further. Hydrogen halides, such as hydrogen chloride (HCl), hydrogen bromide (HBr), and hydrogen iodide (HI), are acidic compounds. When they react with alkenes, they add across the double bond, forming a new single bond.
This reaction is called an addition reaction, and it’s a key concept in organic chemistry. Markovnikov’s rule helps us predict the product of this addition reaction, and it’s especially helpful when dealing with unsymmetrical alkenes, where the two carbon atoms in the double bond have different numbers of hydrogen atoms.
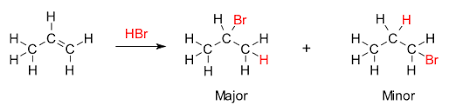
For example, let’s consider the reaction between propene (an unsymmetrical alkene) and hydrogen bromide. Propene has a double bond between the first and second carbon atoms. The first carbon atom has two hydrogen atoms, and the second carbon atom has one hydrogen atom. When hydrogen bromide adds to propene, the hydrogen atom from hydrogen bromide will attach to the second carbon atom (the one with the most hydrogen atoms), and the bromine atom will attach to the first carbon atom (the one with fewer hydrogen atoms).
Markovnikov’s rule also applies to alkene hydration. This is the addition of water (H₂O) to an alkene, and it’s another important reaction in organic chemistry. In this case, the hydrogen from water will attach to the carbon with the most hydrogen atoms, and the hydroxyl group (OH) will attach to the carbon with the fewer hydrogen atoms.
This reaction is often catalyzed by sulfuric acid, which helps to protonate the alkene and make it more reactive. The result is an alcohol, and the specific alcohol formed depends on the structure of the starting alkene.
Understanding Markovnikov’s rule is essential for predicting the products of these important reactions in organic chemistry, and it provides a helpful framework for understanding the behavior of alkenes and hydrogen halides.
See more here: How To Know Markovnikov Vs Anti-Markovnikov? | Difference Between Markovnikov And Anti Markovnikov
What is the difference between Markovnikov rule and anti Markovnikov rule?
Let’s dive into the fascinating world of Markovnikov’s and anti-Markovnikov’s rules! These rules help us predict the outcome of chemical reactions, specifically when adding a protic acid to an unsaturated hydrocarbon (a compound with a double or triple bond).
Markovnikov’s rule tells us that the hydrogen atom from the acid will attach to the carbon atom in the double bond that already has the most hydrogen atoms. This results in the formation of the major product of the reaction.
Anti-Markovnikov’s rule, on the other hand, describes the opposite regioselectivity. It dictates that the hydrogen atom will attach to the carbon atom in the double bond that has fewer hydrogen atoms. This rule applies when we use a radical initiator to promote the reaction.
Think of it like this: Imagine a race between two runners, hydrogen and the acid’s functional group. Markovnikov’s rule says the hydrogen will reach the “finish line” (the carbon with more hydrogen atoms) first. Anti-Markovnikov’s rule suggests the hydrogen will instead go to the carbon with fewer hydrogen atoms, leading to a different product.
Let’s take a look at an example. Consider the addition of hydrogen bromide (HBr) to propene (CH3CH=CH2). According to Markovnikov’s rule, the hydrogen atom from HBr will attach to the terminal carbon, forming 2-bromopropane (CH3CHBrCH3) as the major product. In contrast, using a radical initiator, the anti-Markovnikov’s rule would guide the hydrogen to attach to the middle carbon, resulting in 1-bromopropane (CH2BrCH2CH3) as the major product.
Understanding these rules helps chemists predict the products of chemical reactions and design reactions to synthesize specific compounds.
Key Takeaways:
Markovnikov’s rule focuses on hydrogen attachment to the carbon with more hydrogen atoms, giving the major product.
Anti-Markovnikov’s rule emphasizes hydrogen attachment to the carbon with fewer hydrogen atoms, but this rule requires the use of a radical initiator.
* These rules help chemists predict the outcome of reactions and design synthetic routes to specific compounds.
What is Markovnikov’s rule in chemistry?
Think of it like this: When a protic acid like hydrochloric acid (HCl) reacts with an alkene (a hydrocarbon with a double bond), the hydrogen atom from the acid will preferentially attach itself to the carbon atom in the double bond that already has the most hydrogen atoms. This is the Markovnikov addition.
Now, the anti-Markovnikov rule describes the opposite scenario. It tells us that in some cases, the hydrogen atom might attach itself to the carbon atom with fewer hydrogen atoms. This is a bit less common and often happens when we have special reagents involved, like peroxides.
So, in essence, Markovnikov’s rule gives us a framework to predict the major product of a reaction, while the anti-Markovnikov rule tells us about the minor product, which is less likely to form.
Let’s break down Markovnikov’s rule further. It’s based on the idea that the more substituted carbon atom in a double bond is more stable due to the electron-donating effect of alkyl groups. This stability arises from the fact that alkyl groups are electron-releasing, making the carbon atom more electron-rich.
During the addition reaction, the protic acid acts as an electrophile (electron-seeking species). The electron-rich carbon atom in the double bond attracts the electrophilic hydrogen atom from the acid. This results in the formation of a more stable carbocation, an intermediate species where the carbon atom has a positive charge.
The next step involves the attack of the halide ion (like chloride ion from HCl) on the carbocation, leading to the formation of the final product. The hydrogen atom preferentially attaches to the carbon atom that was already more substituted, making the final product the most stable one.
In conclusion, Markovnikov’s rule provides a useful tool to predict the outcome of many organic reactions. It helps us understand the regioselectivity of addition reactions, which is the preferential addition of a reagent to one particular site in a molecule. Understanding this rule is essential for anyone studying organic chemistry, as it allows us to make informed predictions about the products of reactions and design synthetic strategies accordingly.
Which rule is opposite to Markovnikov’s rule?
Anti-Markovnikov’s rule is often called the peroxide effect or the Kharash effect. It’s named after Morris Kharasch, the chemist who discovered it. This rule describes the addition of a protic acid (HX, where X is a halogen) to an alkene in the presence of a peroxide. What makes Anti-Markovnikov’s rule different from Markovnikov’s rule is that the hydrogen atom gets attached to the carbon that already has more hydrogen atoms, while the halogen atom goes to the carbon that has fewer hydrogen atoms. Think of it as the hydrogen atom choosing the more crowded side!
You might be wondering, why does this happen? Well, it’s all because of the presence of a peroxide. Peroxides are special molecules that initiate a radical reaction. A radical is a species with an unpaired electron, and it’s very reactive. This radical reaction goes through a series of steps that eventually leads to the formation of the Anti-Markovnikov’s product.
Anti-Markovnikov’s rule is a useful tool for chemists because it allows them to control the regioselectivity of reactions, meaning they can choose which product they want to form. This is important in many areas of chemistry, including the synthesis of new drugs and materials.
What is a Markovnikov rule in a hydroboration reaction?
Let’s break it down. Markovnikov’s rule states that when a protic acid (like HBr) adds to an alkene, the hydrogen atom will bond to the carbon atom with the most hydrogen atoms already attached. This means the hydrogen atom will add to the less substituted carbon.
Think of it like this: If you have an alkene with a carbon on one end that’s attached to three hydrogen atoms and a carbon on the other end attached to just one hydrogen, the hydrogen from the acid will add to the carbon with the one hydrogen.
However, hydroboration reactions are different. They are a special type of reaction that does not follow Markovnikov’s rule.
In a hydroboration reaction, boron (B) and hydrogen (H) from a borane (BH3) add across a double bond. The boron atom will attach to the less substituted carbon atom and the hydrogen atom will attach to the more substituted carbon.
So, in hydroboration reactions, Markovnikov’s rule is not followed. Instead of the hydrogen atom adding to the less substituted carbon, it actually adds to the more substituted carbon! This is why it’s important to understand the nuances of these reactions and not just rely on Markovnikov’s rule blindly.
I hope this explanation helps! Let me know if you have any further questions.
See more new information: musicbykatie.com
Difference Between Markovnikov And Anti Markovnikov | What Is The Difference Between Markovnikov Rule And Anti-Markovnikov Rule?
What are Markovnikov and Anti-Markovnikov Reactions?
Imagine an alkene, a molecule with a double bond between two carbon atoms. Now, picture a reagent, like a hydrogen halide (like HCl), wanting to add itself to this double bond. The question is, where does the hydrogen atom go and where does the halide atom go?
This is where Markovnikov’s Rule comes in. It’s like a guiding principle for these addition reactions, stating that the hydrogen atom will attach to the carbon atom that already has more hydrogen atoms. This basically means the hydrogen atom will go to the carbon with the most hydrogens, leading to the more substituted product.
But sometimes, things don’t follow the rule. That’s where Anti-Markovnikov reactions step in. These reactions, as the name suggests, go against the Markovnikov rule, with the hydrogen atom attaching to the carbon with fewer hydrogens, resulting in the less substituted product.
Why does this happen?
The reason for this difference lies in the mechanism of these reactions.
In Markovnikov reactions, the mechanism involves the formation of a carbocation intermediate. This intermediate is a positively charged carbon atom, and its stability depends on the number of alkyl groups attached to it. The more alkyl groups, the more stable the carbocation.
Markovnikov’s Rule arises from the fact that the more stable carbocation is formed when the hydrogen atom attaches to the carbon with more hydrogens, leading to the more substituted product.
Now, let’s look at Anti-Markovnikov reactions. These usually happen in the presence of a radical initiator, such as peroxides. Here, the reaction proceeds via a radical mechanism and the hydrogen atom attaches to the carbon with fewer hydrogens.
What’s the difference between the two?
Here’s a simple table to summarize the key differences between Markovnikov and Anti-Markovnikov reactions:
| Feature | Markovnikov | Anti-Markovnikov |
|——————|——————|———————-|
| Mechanism | Carbocation | Radical |
| Product | More substituted | Less substituted |
| Reagent | Usually HX, H2SO4 | Peroxides (ROOR) |
| Stability | More stable carbocation | More stable radical |
How do I recognize which reaction is happening?
1. Look at the reaction conditions: The presence of peroxides or radical initiators often suggests an Anti-Markovnikov addition.
2. Look at the product: If the product is the more substituted alkane, it’s likely a Markovnikov reaction. If it’s the less substituted alkane, it’s likely Anti-Markovnikov.
Real-world examples
Markovnikov reactions are quite common in organic chemistry. For example, the addition of HBr to propene in the absence of peroxides will result in 2-bromopropane, the more substituted product.
Anti-Markovnikov reactions are also used in various synthetic applications. For example, the addition of HBr to propene in the presence of peroxides will result in 1-bromopropane, the less substituted product.
FAQs
Q: What is a carbocation?
A: A carbocation is a positively charged carbon atom that is formed in the reaction mechanism. Its stability is determined by the number of alkyl groups attached to it. More alkyl groups lead to a more stable carbocation.
Q: What is a radical?
A: A radical is a species with an unpaired electron. Radicals are highly reactive and can initiate chain reactions.
Q: Why is the Anti-Markovnikov product less substituted?
A: The Anti-Markovnikov product is less substituted because the hydrogen atom attaches to the carbon with fewer hydrogens, resulting in a less substituted alkane.
Q: Can I predict the product of any addition reaction?
A: While Markovnikov and Anti-Markovnikov rules provide guidance, predicting the product of all addition reactions isn’t always straightforward. Sometimes, you need to consider other factors, like steric hindrance and the specific reaction conditions.
Q: Where can I learn more about Markovnikov and Anti-Markovnikov reactions?
A: You can find more information in any good organic chemistry textbook or online resources like Khan Academy.
So, there you have it, a breakdown of Markovnikov and Anti-Markovnikov reactions. Remember, understanding these rules will help you understand the reactions of alkenes and the fascinating world of organic chemistry. Keep exploring!
Markovnikov Rule vs. Anti Markovnikov Rule: What’s the Difference?
Markovnikov’s rule predicts the major product in the addition of protic acids to unsaturated hydrocarbons, while the anti-Markovnikov rule describes the Difference Wiki
Markovnikov vs Anti-Markovnikov in Alkene Addition Reactions
As you follow along with my alkene reactions cheat sheet, you’ll notice that many reactions are labeled Mark or Anti-Mark and syn or anti. The difference is Leah4Sci
Markovnikov’s rule Vs Anti-Markovnikov rule: Examples and Easy …
What is Anti-Markovnikov’s rule? Anti-Markovnikov’s rule states that “When HBr is added to an unsymmetrical alkene in presence of traces of organic chemistnotes.com
Anti-Markovnikov Rule vs. Markovnikov Rule – What’s the
Anti-Markovnikov Rule vs. Markovnikov Rule What’s the Difference? The Anti-Markovnikov Rule and the Markovnikov Rule are two contrasting principles that describe the thisvsthat.io
17.2: Markovnikov Orientation vs. Syn or Anti Addition
The term Markovnikov orientation refers to the bonding preference of E and Y for carbon atoms a or b. The following example shows how a proton acid HY can add to the π-bond of an unsymmetrical Chemistry LibreTexts
Markovnikov’s Rule: Statement and Explanation with Examples
Markovnikov’s Rule, also known as Markownikoff’s rule, can be used to describe the outcome of some chemical addition reactions. The Russian chemist Vladimir Vasilyevich BYJU’S
Markovnikov’s Rule – Organic Chemistry Portal
Markovnikov Rule predicts the regiochemistry of HX addition to unsymmetrically substituted alkenes. The halide component of HX bonds preferentially at the more highly Organic Chemistry Portal
10.10: Markovnikov’s Rule – Chemistry LibreTexts
This rule of thumb is known as Markovnikov’s rule, after the Russian chemist Vladimir Markovnikov who proposed it in 1869. While it is useful in many cases, Markovikov’s rule Chemistry LibreTexts
Markovnikov’S Rule | Anti-Markovnikov’S Rule | Mechanism
Markovnikov’S Rule Vs Anti-Markovnikov In Alkene Addition Reactions
Markovnikov’S Rule
Markovnikov And Anti Markovnikov Rule [Complete] In Just 15 Minutes | Organic Chemistry
Difference Between Markovnikov’S And Anti Markovnikov’S Rule With Notes.
#Tips On Markovnikov’S And Anti-Markovnikov’S (Peroxide Effect)
Anti-Markovnikov Hydrohalogenation
Markovnikov’S Rule And Carbocations | Alkenes And Alkynes | Organic Chemistry | Khan Academy
Link to this article: difference between markovnikov and anti markovnikov.
See more articles in the same category here: https://musicbykatie.com/wiki-how/
