Table of Contents
How are cross bridges formed?
Think of it like this: The myosin head acts like a tiny, powerful arm, and it’s always eager to grab hold of actin. When it does, it flexes, pulling the actin filament closer to the myosin filament. This flexing movement is called a power stroke. The power stroke is what generates the force that allows your muscles to contract.
Now, let’s delve deeper into the process. For the myosin head to grab onto actin, it needs a little help. That help comes in the form of calcium ions. When you decide to move a muscle, your nervous system sends a signal to your muscle fibers. This signal triggers the release of calcium ions. These calcium ions bind to a protein called troponin on the actin filament. When troponin binds to calcium, it moves another protein called tropomyosin out of the way. Tropomyosin normally blocks the binding sites on actin where the myosin head wants to attach. Once tropomyosin is out of the way, the myosin head can finally grab onto the actin filament and initiate the power stroke.
The myosin head doesn’t just hold onto actin forever. It actually needs to detach and re-attach to keep the muscle contracting. This detachment occurs when a molecule called ATP (adenosine triphosphate) binds to the myosin head. ATP provides the energy for the myosin head to detach and then re-attach further down the actin filament. This cycle of attachment, detachment, and re-attachment continues as long as there are calcium ions present and ATP available.
In essence, the process of cross-bridge formation is a carefully orchestrated dance between actin, myosin, calcium, troponin, tropomyosin, and ATP. It’s a beautiful example of how biological systems use complex interactions to produce powerful results.
What needs to bind to the myosin head to break the cross-bridge?
Let’s dive deeper into how ATP works to break the cross-bridge and allow for muscle relaxation. Imagine a muscle fiber, made up of many tiny filaments called actin and myosin. These filaments are arranged in a way that allows them to slide past each other, which is what causes muscle contraction.
When a muscle is relaxed, the myosin head is not attached to the actin filament. However, when the muscle receives a signal to contract, calcium ions are released, allowing the myosin head to bind to the actin filament, forming a cross-bridge. This binding initiates the “power stroke,” a movement of the myosin head that pulls the actin filament toward the center of the sarcomere, causing the muscle to shorten.
ATP plays a crucial role in this process. Once the myosin head binds to the actin filament, it needs ATP to detach. ATP binds to the myosin head, triggering a change in its conformation. This change weakens the bond between the myosin head and the actin filament, causing the cross-bridge to break. Once the cross-bridge is broken, the myosin head can then bind to a new actin filament, ready for another power stroke.
In essence, ATP acts like a key that unlocks the myosin head from the actin filament, allowing the muscle to relax. Without ATP, the cross-bridge would remain intact, preventing the muscle from relaxing and leading to a state of rigor mortis.
What is the binding site for myosin cross bridges?
Now, myosin has another trick up its sleeve – it can also bind to ATP. This is like fuel for myosin; it gives it the energy it needs to move. When ATP binds to myosin, it’s like turning on a little motor inside myosin. This motor breaks down ATP into ADP and a phosphate molecule. This releases energy that allows myosin to detach from actin. It’s a bit like unplugging the motor!
So, to sum it up, the binding site on actin is where myosin hooks on, and the ATP binding site on myosin is where the energy comes from to make myosin move.
The Myosin Binding Site: A Closer Look
Think of actin like a long train track. This track is made of repeating units of actin molecules, each with a myosin binding site. The myosin cross-bridge is like a train engine. It can attach to the track (actin) at these binding sites and pull the train (filaments) forward.
But how does the engine know where to attach?
Well, the binding site on actin is specifically designed to fit the myosin cross-bridge. It’s like a lock and key: only the right key (myosin) can unlock the door (the binding site). This ensures that the myosin cross-bridge attaches to the actin filament in the correct position for muscle contraction.
Here’s the really cool part: the binding site on actin can actually change its shape. This is important because it allows the muscle to relax and contract. When the muscle is relaxed, the binding site on actin is blocked by tropomyosin, another protein. This prevents the myosin cross-bridge from attaching. But when the muscle needs to contract, calcium ions bind to troponin, a protein that sits on tropomyosin. This causes tropomyosin to move out of the way, revealing the binding site on actin so that myosin can attach and pull.
It’s a fascinating dance of proteins, shapes, and energy that allows our muscles to move!
What causes myosin to form a cross-bridge with actin?
Let’s break down why ATP is so important. Imagine myosin as a little motor protein that wants to move along actin, which is like a track. Myosin can’t just jump onto the track; it needs energy to get going. This energy comes from ATP. When ATP binds to myosin, it changes the shape of myosin, allowing it to attach to the myosin-binding sites on actin. This is the start of the cross-bridge cycle.
Now, the cross-bridge cycle is a series of events that happen repeatedly, and it’s what makes muscle contraction possible. Here’s a simplified version of what happens:
1. ATP binds to myosin, causing it to detach from actin.
2. Myosin splits ATP into ADP and a phosphate group, releasing energy.
3. This energy allows myosin to bind to a new myosin-binding site on actin and pull the actin filament closer.
4. The phosphate group detaches from myosin, and myosin releases ADP and returns to its original position.
This cycle keeps repeating as long as there is ATP available and calcium ions are bound to troponin. Think of it like a little engine that keeps chugging along as long as it has fuel (ATP) and a spark (calcium ions). This continuous cycle of attaching, pulling, and detaching is what causes the shortening of the muscle fiber and the force of muscle contraction.
What regulates cross-bridge formation?
Imagine tropomyosin as a long, thin protein that wraps around the actin filaments. It acts like a gatekeeper, blocking the myosin binding sites on the actin filaments, preventing cross-bridge formation. This blockage prevents muscle contraction from occurring randomly.
Troponin is a complex of three proteins that attach to both tropomyosin and actin. This complex acts as a sensor for calcium ions. When calcium ions bind to troponin, they trigger a conformational change in tropomyosin. Tropomyosin then shifts away from the myosin binding sites on actin, allowing myosin to bind and form cross-bridges.
This shift is crucial because it allows the myosin heads to bind to the actin filaments, initiating the sliding filament mechanism that powers muscle contraction. So, when calcium levels are low, tropomyosin blocks the myosin binding sites, keeping the muscle relaxed. However, when calcium levels rise, the troponin-tropomyosin complex shifts, exposing the myosin binding sites, and enabling muscle contraction.
This intricate dance between calcium ions, tropomyosin, and troponin allows for precise control of muscle contraction. It’s a fine-tuned system that ensures muscles only contract when needed, and allows for a smooth, coordinated movement.
What is the effect of ATP binding to myosin?
ATP binding to myosin has a powerful effect: it causes the myosin head to detach from actin. This detachment is crucial because it allows the myosin head to reposition itself and prepare for the next cycle of muscle contraction.
Think of it like this: imagine a tiny hand gripping a bar. To let go, you need to provide the hand with some energy. That’s where ATP comes in. When ATP binds to the myosin head, it provides the energy needed to break the grip on actin.
Now, here’s the cool part. After detaching, the myosin head undergoes a conformational change. This change is triggered by the hydrolysis of ATP into ADP and inorganic phosphate. The myosin head changes shape, effectively “cocking” itself like a spring.
This “cocked” myosin head is now ready to rebind to actin, but this time, it binds to a new position on the actin filament, further along the filament. This movement is what causes the thin filament to slide past the thick filament, shortening the sarcomere and ultimately contributing to muscle contraction.
In essence, ATP binding to myosin is like a signal that triggers the myosin head to release its grip on actin, re-energize itself, and get ready to bind to a new spot on the actin filament. This repeated cycle of binding, detaching, and rebinding is what drives the sliding filament mechanism, ultimately leading to the powerful force of muscle contraction.
What are the two things necessary for cross-bridge formation?
Calcium (Ca2+) ions and ATP are the two crucial players in this process. Think of it like this: calcium is the key that unlocks the door, and ATP is the energy that powers the action.
Here’s how it works:
Calcium’s Role: When an action potential arrives at the muscle fiber, it triggers the release of calcium from the sarcoplasmic reticulum. Calcium then binds to a protein called troponin, which is attached to another protein called tropomyosin. This binding action moves tropomyosin out of the way, exposing the binding sites on the actin filament. This is the key unlocking the door!
ATP’s Role: Now, myosin, the motor protein of muscle contraction, can bind to actin. But to do this, myosin needs to be in its energized state. ATP provides that energy. When ATP is broken down, myosin is cocked, like a spring ready to be released. This allows it to bind to actin and initiate the power stroke which pulls the actin filament closer to the myosin filament, causing the muscle to contract.
It’s a delicate dance between these two essential molecules. Without calcium, the binding sites on actin remain hidden, and myosin cannot attach. Without ATP, myosin cannot be energized and will not bind to actin. So, both are absolutely necessary for cross-bridge formation to occur and for muscle contraction to happen.
Let me illustrate this with a simple analogy. Imagine you have a door with a lock and a handle. The calcium is like the key that unlocks the door, allowing the myosin (imagine this as your hand) to grab the actin (the handle). The ATP is like the energy you need to pull the handle and open the door, causing the muscle to contract.
See more here: What Needs To Bind To The Myosin Head To Break The Cross-Bridge? | Cross Bridges Are Created When Myosin Heads Bind To
See more new information: musicbykatie.com
Cross Bridges Are Created When Myosin Heads Bind To Actin Filaments
Cross bridges are created when myosin heads bind to actin filaments, which are the protein filaments that make up our muscles. Imagine these filaments like tiny ropes, and the myosin heads are like little hooks that grab onto those ropes.
But before we get into the nitty-gritty of the binding process, let’s back up a bit and understand the players involved.
Actin Filaments: The Building Blocks
Actin filaments are thin, long, protein fibers that form the basic structure of muscle cells. They’re like the foundation of our muscles, giving them their shape and structure.
Myosin Heads: The Movers and Shakers
Myosin heads are globular protein structures located on myosin filaments. Think of them as the engines of muscle contraction. They have the ability to bind to actin filaments and pull them closer together, causing muscle fibers to shorten and contract.
The Binding Process: A Step-by-Step Guide
Here’s a step-by-step breakdown of how cross bridges are formed and how they help our muscles contract:
1. Calcium Ions: The Trigger
Everything starts with calcium ions (Ca²⁺). Calcium plays a crucial role in muscle contraction, and its presence sets the whole process in motion.
When a nerve impulse reaches a muscle cell, it causes the release of calcium ions from storage compartments within the muscle cell. These calcium ions then flood the cell, initiating the formation of cross bridges.
2. Binding Sites: The Keyholes
Now, imagine actin filaments have little “keyholes” called binding sites. These binding sites are where the myosin heads attach. But there’s a catch – these binding sites are normally blocked by a protein called tropomyosin.
3. Tropomyosin: The Gatekeeper
Tropomyosin acts like a gatekeeper, blocking the binding sites on actin filaments. This blockage prevents myosin heads from attaching and prevents muscle contraction from happening randomly.
4. Calcium’s Role: The Key
Calcium ions come in to play here. When calcium ions bind to another protein called troponin (which is attached to tropomyosin), this causes a conformational change in tropomyosin. Imagine tropomyosin shifting its position, moving away from the binding sites on actin filaments and opening the “gate” for the myosin heads to attach.
5. Myosin Heads: The Hooks
Now that the binding sites are open, the energized myosin heads can finally attach themselves to the actin filaments. This attachment forms the cross bridge.
Think of it like this: the myosin heads have been waiting in the wings, ready to grab hold of the actin filaments. Once the binding sites are open, the myosin heads can finally connect and start pulling!
6. Power Stroke: The Pull
Once the myosin head binds to the actin filament, it goes through a conformational change, pivoting forward and pulling the actin filament closer to the center of the sarcomere (the basic unit of a muscle fiber). This is called the power stroke.
7. Detachment and Reattachment: The Cycle Continues
The myosin head then detaches from the actin filament and returns to its original position. This cycle of attachment, pulling, and detachment repeats as long as calcium ions are present, leading to sustained muscle contraction.
As long as calcium ions are available, the myosin heads can continue to attach, pull, detach, and reattach, resulting in a continuous shortening of the muscle fiber and a sustained contraction.
Breaking Down the Terminology
Let’s recap the key terms we’ve discussed:
Cross Bridge: The connection between a myosin head and an actin filament
Actin Filament: A thin protein fiber that forms part of the muscle structure
Myosin Filament: A thick protein fiber that contains myosin heads
Myosin Head: The globular protein structure responsible for binding to actin filaments and pulling them closer together
Binding Site: The specific location on an actin filament where a myosin head can attach
Tropomyosin: A protein that blocks binding sites on actin filaments, preventing random muscle contraction
Troponin: A protein that binds to calcium ions and causes tropomyosin to shift, exposing the binding sites
Sarcomere: The basic unit of a muscle fiber, containing actin and myosin filaments
Power Stroke: The movement of the myosin head as it pulls the actin filament closer to the center of the sarcomere
The Importance of Cross Bridges
Cross bridges are essential for muscle contraction. They are the driving force that allows us to move our bodies, lift weights, and perform all sorts of physical activities. Without them, our muscles wouldn’t be able to generate the force needed for movement.
Let’s Address Some Common Questions
Here are some frequently asked questions about cross bridges:
1. What happens when cross bridges are broken?
When calcium ions are removed from the muscle cell, tropomyosin returns to its blocking position, covering the binding sites on actin filaments. This prevents the myosin heads from attaching and stops muscle contraction.
2. What are some factors that can affect cross bridge formation?
Several factors can affect cross bridge formation and muscle contraction, including:
Calcium Concentration: Higher calcium levels lead to more cross bridges forming.
ATP Availability:Myosin heads require ATP (adenosine triphosphate) for energy to bind to actin filaments.
Temperature: Increased temperature can affect the rate of cross bridge formation and muscle contraction.
pH: Extreme pH changes can interfere with the function of the proteins involved in cross bridge formation.
3. Can cross bridge formation be influenced by certain medications?
Yes! Some medications, like those used to treat muscle disorders or heart conditions, can affect cross bridge formation. For example, certain medications can interfere with the release of calcium ions or block the binding of myosin heads to actin filaments.
4. What are some examples of cross bridges in action?
Think about all the movements you do every day! From walking, running, and lifting weights to typing on a keyboard and chewing your food, cross bridges are constantly working behind the scenes to make it all possible.
5. How can I improve my muscle strength and cross bridge formation?
Regular exercise is key to building muscle strength. Strength training involves lifting weights or performing exercises that challenge your muscles, which can lead to increased protein synthesis, muscle fiber growth, and ultimately, more cross bridges being formed.
6. What happens to cross bridges when we get older?
As we age, our muscle mass naturally decreases, and this can affect cross bridge formation. This is why it’s important to stay active as we get older to help maintain muscle mass and function.
Understanding cross bridges is key to understanding how our muscles work. It’s a complex process involving many different proteins and molecules, but it’s amazing how these tiny components come together to allow us to move our bodies. Now that you know how cross bridges are formed, you can appreciate the incredible complexity and efficiency of our muscular system.
Cross Bridge Cycle: Flashcards | Quizlet
cross bridge formation:Activated myosin head binds to actin forming a cross bridge; Inorganic phospahte released; Bond between myosin and actin becomes stronger Quizlet
ATP and Muscle Contraction – Biology LibreTexts
Once the myosin forms a cross-bridge with actin, the Pi disassociates and the myosin undergoes the power stroke, reaching a lower energy state when the Biology LibreTexts
Sliding Filament Theory, Sarcomere, Muscle Contraction, Myosin
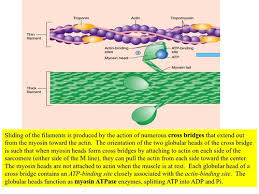
As the myosin S1 segment binds and releases actin, it forms what are called cross bridges, which extend from the thick myosin filaments to the thin actin filaments. Nature
Cross bridge-cycling – Basic Human Physiology
Cross-bridge formation occurs when the myosin head attaches to actin while ADP and Pi are still bound to myosin. Pi is then released, causing the myosin head to move toward Pressbooks
Cross-bridge Cycle During Muscle Contraction (Video) | JoVE
At the molecular level, this is a cyclic, multistep process that involves binding and hydrolysis of ATP, and movement of actin by myosin. When ATP, that is attached to the myosin JoVE
10.2 Skeletal Muscle – Anatomy & Physiology
Tropomyosin winds around the chains of the actin filament and covers the myosin-binding sites to prevent actin from binding to myosin. The troponin-tropomyosin complex uses calcium ion binding to TnC to Open Educational Resources
ATP and Muscle Contraction | Biology for Majors II
Tropomyosin blocks myosin binding sites on actin molecules, preventing cross-bridge formation and preventing contraction in a muscle without nervous input. Troponin binds to tropomyosin and helps to position it Lumen Learning
Muscle Contraction and Locomotion | OpenStax
The number of cross-bridges formed between actin and myosin determine the amount of tension that a muscle fiber can produce. Cross-bridges can only form where thick and thin filaments overlap, allowing Lumen Learning
Muscle Contraction – Cross Bridge Cycle, Animation.
Muscle Tissues And Sliding Filament Model
Muscle Contraction – Cross Bridge Cycle
5. Details Of Actin-Myosin Crosslinking
Muscle Contraction 3D
Crossbridge Cycle: Muscle Contraction
Muscle Contraction In 60 Seconds! #Shorts
Muscle Lecture 6, Crossbridge Cycling With Atp
Link to this article: cross bridges are created when myosin heads bind to.
See more articles in the same category here: https://musicbykatie.com/wiki-how/
