Table of Contents
Is a non-metal an insulator?
Think of it like this: imagine a playground with kids playing. In a metal, the kids are running around freely, bumping into each other and easily passing a ball (representing the electrons). But in a non-metal, the kids are tightly holding onto their toys (electrons) and are less likely to share or pass them around. This makes it difficult for the ‘ball’ of electricity to travel through the non-metal.
While most non-metals are excellent insulators, there are a few exceptions. For example, graphite, a form of carbon, is a good conductor of electricity. This is because its structure allows for electrons to move freely between its layers.
Another interesting fact is that some non-metals can actually be made into semiconductors. These materials have properties that fall somewhere between conductors and insulators. They can be used to create electronic devices like transistors and diodes, which are essential components of modern electronics.
So, while most non-metals are insulators, it’s important to remember that there are always exceptions to the rule!
Are all non-metals insulators True or false?
However, it’s important to remember that not all non-metals are perfect insulators. Some non-metals, like graphite, can actually conduct electricity quite well. This is because graphite has a unique structure where electrons can move freely between layers of carbon atoms.
So, while it’s a good general rule that non-metals are insulators, there are always exceptions.
Let’s delve a little deeper into why non-metals are generally good insulators.
Think of the atoms in a material like a bunch of tiny balls connected by springs. In metals, these springs are very loose, allowing the balls (electrons) to move freely and conduct electricity. But in non-metals, the springs are much tighter, making it harder for the electrons to move around. This means electricity can’t flow through them easily.
To put it another way, non-metals have a high resistance to the flow of electricity. This means that it takes a lot of energy to force electricity through them. This resistance is what makes them useful as insulators, protecting us from dangerous electric currents.
But, as we mentioned, graphite is a bit of a rebel in the non-metal world. It’s made of layers of carbon atoms arranged in a unique hexagonal structure. This structure allows electrons to move freely within each layer, making graphite a decent conductor of electricity.
So, the next time you hear someone say that all non-metals are insulators, you can politely remind them that graphite is a shining example of a non-metal that bucks the trend!
Are all non-metals conductors?
Let’s break down why some nonmetals conduct electricity:
Graphite: In graphite, carbon atoms are arranged in sheets with strong bonds within each sheet but weak bonds between them. This structure allows electrons to move freely within the sheets, making graphite a good conductor of electricity.
Silicon: Silicon is a semiconductor, meaning it can act as both a conductor and an insulator. Its conductivity depends on factors like temperature, impurities, and the presence of an electric field. Silicon’s unique properties make it crucial in electronic devices like transistors and computer chips.
Metalloids: Metalloids occupy a middle ground between metals and nonmetals on the periodic table. Their conductivity can vary depending on factors like temperature and impurities.
The key takeaway is that while many nonmetals are insulators, some exceptions exist with unique properties that allow them to conduct electricity. This variety of behavior is what makes nonmetals fascinating and essential in many technological applications.
Are all non conductors insulators?
While all insulators are non-conductors, not all non-conductors are insulators. This is because some materials, like semiconductors, can conduct electricity under specific conditions. For example, semiconductors can conduct electricity when exposed to light or when heated.
Think of it like this:
Insulators are like closed doors, completely blocking the flow of electricity.
Non-conductors are like doors that might be partially open or have a latch that can be opened under the right circumstances, allowing a limited flow of electricity.
Semiconductors play a crucial role in modern electronics. They are used in devices like transistors, diodes, and integrated circuits, allowing us to control the flow of electricity in a precise way. This ability to control electricity under specific conditions makes them essential for building complex electronic systems.
So, while insulators are a specific type of non-conductor that completely blocks electricity, not all non-conductors are insulators. The ability of some non-conductors to conduct electricity under certain conditions opens up a world of possibilities in electronics and beyond.
Can an insulator be metal?
Hemley and Naumov discovered something really interesting: for a metal to become an insulator, the spaces between its atoms have to be organized in a specific way. This kind of asymmetry wasn’t understood before their work. When this happens, the electrons get stuck between the atoms, and they can’t flow freely anymore. This is how a metal can become an insulator.
To understand this better, imagine a bunch of marbles in a box. If the marbles are all close together, they can roll around easily, representing the free movement of electrons in a metal. But if you arrange the marbles in a pattern where they’re separated by barriers, they can’t move as freely. This represents how a metal can become an insulator.
So, what are some examples of this? One fascinating example is cesium, which is a metal under normal conditions. But, if you put cesium under extremely high pressure, it transforms into an insulator! This is because the pressure squeezes the cesium atoms closer together, creating the specific kind of asymmetry that Hemley and Naumov described. As a result, the electrons become localized and the cesium becomes an insulator.
This might sound a bit complicated, but it’s a great example of how scientists are learning about the amazing properties of materials. By understanding these properties, we can create new and exciting technologies, like improved solar cells or even new types of electronics!
Which metal is not an insulator?
Let’s break down why mercury is unique. Most metals are great conductors of electricity. This is because they have free electrons that can easily move around and carry an electrical current. Mercury, however, is a bit different. It’s a liquid at room temperature, and its atoms are arranged in a way that makes it harder for electrons to flow freely.
Think of it like this: in a solid metal, the atoms are tightly packed together, allowing electrons to move easily from one atom to another. In mercury, the atoms are further apart, making it harder for electrons to hop between them. This means mercury doesn’t conduct electricity as well as other metals.
Even though mercury doesn’t conduct electricity well, it’s still considered a metal. It has all the other properties of metals, like being shiny and malleable. It’s just a bit of an oddball when it comes to electrical conductivity.
Are all metals good insulators?
What about insulators?
Insulators, on the other hand, are materials that resist the flow of heat and electricity. They do this by holding their electrons tightly, preventing them from moving freely. This makes them excellent for applications where you need to prevent the flow of energy, such as in electrical wiring insulation or heat-resistant materials.
Metals and Insulators: A Tale of Two Types
So, are all metals good conductors? The answer is a bit more nuanced. While most metals are indeed excellent conductors, there are exceptions. Some metals, like lead and mercury, are actually poorer conductors than others. This is because their electrons are held a bit more tightly, making them less able to move freely.
The Role of Free Electrons
The key to understanding why some metals are better conductors than others lies in the number of free electrons they possess. Metals with a higher concentration of free electrons are typically better conductors. This is why copper, with its abundance of free electrons, is a top choice for electrical wiring.
Understanding the Exceptions
It’s also important to remember that the conductivity of a metal can be influenced by factors like temperature and impurities. As temperature increases, the atoms in a metal vibrate more, making it harder for electrons to move freely. Similarly, the presence of impurities can disrupt the flow of electrons, reducing conductivity.
In short, while most metals are excellent conductors, there are exceptions to this rule. The conductivity of a metal depends on several factors, including the number of free electrons, temperature, and impurities. So, it’s not always a straightforward “yes” or “no” when it comes to metals and conductivity.
Are all metalloids insulators?
Metalloids are a group of elements that share characteristics of both metals and nonmetals. One of the key features that sets them apart is their electrical conductivity. Unlike metals, which are excellent conductors of electricity, metalloids have a special trick up their sleeve – they can act as semiconductors.
This means that their ability to conduct electricity can be controlled, making them extremely useful in electronics. Think of it like a switch that can be flipped on or off, allowing them to act as either a conductor or an insulator.
This incredible ability depends on a couple of factors:
Impurities: Just like adding a pinch of salt to water changes its boiling point, adding tiny amounts of other elements (impurities) to a metalloid can dramatically change its conductivity.
Temperature: Just like a hot day makes you sweat more, raising the temperature of a metalloid can make it more conductive.
This “adjustable” electrical behavior makes metalloids the heroes of the electronics world. They are essential in making transistors, solar cells, and countless other devices that power our modern lives.
So, to answer your question, not all metalloids are insulators. They can act as both conductors and insulators, depending on the circumstances. They are the chameleons of the periodic table, adapting their electrical behavior to the needs of the moment.
Is there a true insulator?
Think about it this way: Imagine insulation as a super strong wall. It can block a lot of tiny things trying to get through, like little ants. But if you throw a giant boulder at that wall, it’s going to crack, right? That giant boulder is like a high voltage.
So, even the best insulators will eventually break down if you apply enough voltage. That’s because, even though they’re very good at resisting the flow of electricity, they’re not completely impenetrable. Some tiny amount of current can still flow through them, even at low voltages. It’s just a really, really small amount.
It’s like a tiny crack in the wall. It might be so small you can’t see it, but if you push hard enough, it will eventually break. That’s how insulators work. They’re not perfect, but they’re incredibly good at their job – keeping that electricity flowing where it’s supposed to be and stopping it from going where it shouldn’t.
Is it true that all metals are conductors?
The ability of a metal to conduct electricity comes down to how easily electrons can move through its structure. Metals have a unique structure where their outermost electrons are loosely bound to the atoms. These electrons are free to roam throughout the material, acting like tiny charge carriers. When a voltage is applied, these free electrons flow, creating an electrical current.
The conductivity of a metal depends on several factors:
The number of free electrons: Metals with more free electrons generally conduct better.
The temperature: As temperature increases, the atoms vibrate more, making it harder for electrons to flow freely. This leads to reduced conductivity.
The purity of the metal: Impurities in a metal can act like obstacles, hindering the flow of electrons.
The crystal structure: The arrangement of atoms in a metal’s structure can also influence conductivity.
But wait, there are metals that don’t conduct electricity very well! Think of mercury. It’s a liquid metal at room temperature but is a poor conductor compared to others. Then there’s manganese, which is a relatively poor conductor too.
So, while it’s true that most metals are good conductors, it’s not universally true for all metals. It’s important to understand the factors that contribute to a metal’s conductivity and keep in mind that there are exceptions to every rule.
See more here: Are All Non-Metals Insulators True Or False? | Are All Non Metals Insulators
What is a nonmetal insulator?
Imagine electrons as tiny, energetic particles zipping around inside a material. In nonmetal insulators, these electrons are tightly bound to their atoms. They don’t move freely, making these materials excellent at resisting the flow of electricity. Think of it like a crowded room – people can’t move around easily.
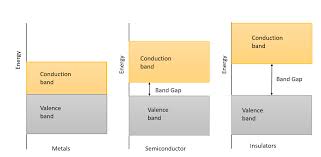
Now, let’s talk about activation energy and band gap. These terms describe how much energy an electron needs to break free from its atom and start conducting electricity. In nonmetal insulators, the activation energy is high, meaning it takes a lot of energy to get those electrons moving. This is like a high wall you have to climb to get into a party. It’s pretty tough to get over!
Nonmetal insulators have a wide band gap – a large gap between the energy levels where electrons can exist. This means electrons have to gain a lot of energy to jump from one level to another and contribute to conductivity.
Here’s the cool thing: as the temperature rises, electrons get more energized and can overcome the activation energy more easily. This means that the conductivity of nonmetal insulators increases slightly as the temperature goes up. It’s like more people are willing to climb that wall when it gets warmer!
However, if the voltage or temperature gets too high, the material can break down. This means the insulator suddenly starts conducting electricity, which can be a problem in many applications. Think of it like the party wall suddenly collapsing when too many people try to climb over it.
Nonmetal insulators are super important in our daily lives. They help us control and isolate electricity safely. You can find them in all sorts of things, like the plastic casing of your phone or the glass in your light bulb.
Is a conductor a good insulator?
Metals, like copper, are excellent conductors because they have free electrons that can easily move and carry an electric current. Non-metallic solids, on the other hand, are usually good insulators. These materials have tightly bound electrons that are difficult to move, making it hard for electricity to flow through them.
To understand why conductors and insulators behave this way, we need to look at their atomic structure. Conductors have a structure where electrons can move freely from atom to atom. This happens because the outermost electrons in conductors are loosely bound to the atom and can easily break free. These free electrons are called conduction electrons, and they are the reason why electricity can flow easily through conductors.
Insulators, on the other hand, have tightly bound electrons. These electrons are held very close to the nucleus of the atom and require a lot of energy to break free. As a result, electricity cannot flow easily through insulators.
Here are some examples of conductors and insulators:
Conductors:
Copper
Aluminum
Gold
Silver
Iron
Insulators:
Rubber
Glass
Plastic
Wood
Ceramic
So, to answer your question, a conductor is not a good insulator. In fact, it’s the opposite. Conductors allow electricity to flow freely, while insulators resist the flow of electricity.
Let’s break down this concept further:
Conductors have free electrons that can easily move and carry an electric current. Think of these free electrons as like tiny messengers that can quickly pass information (electricity) along.
Insulators have tightly bound electrons that are difficult to move. These electrons are like messengers that are stuck in place and can’t pass information along easily.
This difference in electron behavior is what makes the difference between a conductor and an insulator.
What makes a material a conductor or insulator?
Conductors, like iron and steel, are materials where electrons can flow freely. Imagine them like a busy highway with lots of lanes for cars (electrons) to travel easily. This is because they have loosely bound electrons in their outer shell, ready to move around and carry an electrical current.
Insulators, like glass and plastic, are the opposite. Their electrons are tightly bound to their atoms, making it very difficult for them to move. It’s like a narrow, congested road with lots of traffic jams – electrons can’t flow easily.
Think about it this way: if you want to get a current flowing, you need a material where electrons can move easily. That’s why conductors are so important in electrical circuits. They allow electricity to travel from one point to another, powering our devices. Insulators are equally important, as they keep electricity confined to its intended path, preventing dangerous shocks and short circuits. They’re like the walls and barriers that keep the electrical current flowing safely within its designated path.
What is an electrical insulator?
Why do insulators act this way? Well, it all boils down to the electrons in their atoms. Insulators have electrons that are tightly bound to their atoms. This means they don’t easily break free and move around, which is what allows electricity to flow. Semiconductors are somewhere in between conductors and insulators, and they can be made to act like either depending on how they’re treated.
Think of it like this: imagine a playground with kids. Conductors are like a playground with kids who love to run around and play tag, easily moving from one place to another. Insulators are like a playground with kids who prefer to stay close to their parents, not wanting to wander far. And semiconductors are like kids who sometimes want to play tag and sometimes prefer to stay close to their parents.
So, insulators are important because they keep electricity contained where it needs to be. This is crucial in many applications, like keeping electrical wires from shocking us or preventing electricity from leaking out of electrical devices. They’re the unsung heroes of the electrical world!
See more new information: musicbykatie.com
Are All Nonmetals Insulators: A Closer Look
What are Nonmetals?
Nonmetals are a category of elements on the periodic table that share certain characteristics. They tend to be brittle (they break easily), dull (they don’t shine), and are generally poor conductors of heat and electricity. Think about sulfur, phosphorus, oxygen, and carbon. These are all nonmetals.
What are Insulators?
Now, what makes a material an insulator? An insulator is a material that resists the flow of electricity. It’s like a traffic jam for electrons – they don’t flow easily through it. This is because insulators have tightly bound electrons that don’t move freely.
The Relationship Between Nonmetals and Insulators
So, why are many nonmetals insulators? It boils down to the structure of their atoms. Nonmetals usually have a full outer shell of electrons. These electrons are tightly bound to the atom and are not easily freed to carry an electric current.
The Exceptions
But, like I said earlier, there are exceptions.
Graphite: Graphite is a form of carbon and a nonmetal, but it’s a good conductor of electricity. This is because graphite has a unique structure where the carbon atoms are arranged in sheets, and within these sheets, some electrons are free to move.
Silicon: Silicon is also a nonmetal, but it can be made into a semiconductor. Semiconductors are materials that can act as both conductors and insulators, depending on certain conditions. This makes silicon incredibly important in electronics!
Why are these Exceptions Important?
These exceptions show us that the relationship between nonmetals and insulators isn’t always straightforward. It’s important to remember that the properties of materials are based on their atomic structure.
So, are all nonmetals insulators?
The simple answer is no. While many nonmetals are good insulators, there are exceptions like graphite and silicon. These exceptions highlight how complex the world of chemistry and materials science really is.
FAQs
Q: What are some common examples of nonmetals that are good insulators?
A: Think about everyday materials like rubber, glass, wood, and plastic. These are all made up of nonmetal elements and are excellent insulators.
Q: Why is graphite a conductor?
A: Graphite has a unique layered structure. Within these layers, electrons are able to move freely, making it a good conductor of electricity.
Q: What makes silicon a semiconductor?
A: Silicon’s ability to act as both a conductor and an insulator comes from its unique atomic structure and the way it can be modified by doping with other elements.
Q: Are there other nonmetal exceptions to the insulator rule?
A: While graphite and silicon are the most commonly known examples, there are a few others. For example, selenium is a nonmetal that can also be made into a semiconductor.
Remember: While nonmetals are generally good insulators, there are always exceptions to the rule!
10 Examples of Electrical Conductors and Insulators
Simply put, electrical conductors are materials that carry (or conduct) electrical currents well, such as iron and steel, and insulators ThoughtCo
Conductors and Insulators – HyperPhysics
In a conductor, electric current can flow freely, in an insulator it cannot. Metals such as copper typify conductors, while most non-metallic solids are said to be good insulators, HyperPhysics
Electricity – Conductors, insulators, and semiconductors
Electricity – Conductors, insulators, and semiconductors: Materials are classified as conductors, insulators, or semiconductors according to their electric conductivity. The classifications can be Britannica
Nonmetal | Definition, Properties, Examples, & Facts
This means that nonmetals display low (insulators) to moderate (semiconductors) bulk electrical conductivities, which increase with increasing temperature, and are subject to dielectric breakdown at Britannica
Physics Tutorial: Conductors and Insulators
Examples of insulators include plastics, Styrofoam, paper, rubber, glass and dry air. The division of materials into the categories of conductors and insulators is a somewhat artificial division. It is more appropriate to The Physics Classroom
Choosing and using conductors and insulators (non-statutory)
Only metals are electrical conductors. All non-metals are electrical insulators. Children will be taught some non-metals are poor insulators, e.g. water as it can contain Oak National Academy
7.3: Conductors and Insulators – Physics LibreTexts
These are called insulators. Electrons and ions in insulators are bound in the structure and cannot move easily—as much as 1023 10 23 times more slowly than in Physics LibreTexts
Conductors and insulators – KS3 Physics – BBC Bitesize
Conductors are materials which allow electrical current to flow through them easily. Metals are generally good electrical conductors. Insulators are materials which are poor BBC
Lesson: Electrical conductors and insulators | KS2 Science | Oak …
Only metals are electrical conductors. All non-metals are electrical insulators. Water is an electrical conductor. Children will test materials for conductivity, but the teaching slides Oak National Academy
What Are Conductors \U0026 Insulators? Are All Metals Conductors? Are All Non-Metals Insulators? (Nso)
Conductors \U0026 Non-Conductors | Properties Of Matter | Chemistry | Fuseschool
Conductors And Insulators – Examples, Definition, Properties | Video For Kids
Lecture 22: Metals, Insulators, And Semiconductors
Electrical Conductors And Insulators
Difference Betweeen A Metal And An Insulator
Form 2 | Science Pt3| Heat Conductors And Heat Insulators
Types Of Insulators, Insulator, Electrical Insulators, Types Of Insulators Used In Overhead Lines
Link to this article: are all non metals insulators.
See more articles in the same category here: https://musicbykatie.com/wiki-how/
