Table of Contents
Is cis-trans isomerism possible in alkynes?
You’re right, alkynes can’t have cis-trans isomers. Think of it this way: a carbon atom involved in a triple bond can only form one additional bond. This means the geometry around the triple bond is linear, and there are no distinct “sides” for the substituents to be on.
Let’s dive a bit deeper into why this is the case. Remember, cis-trans isomerism arises when there’s restricted rotation around a double bond. This restriction is due to the presence of a pi bond, which prevents free rotation. In the case of alkynes, the triple bond contains two pi bonds in addition to a sigma bond. The rigidity of the two pi bonds forces the carbons involved in the triple bond and their attached substituents into a straight line, preventing the possibility of cis or trans arrangements.
Here’s a simple analogy: imagine a stick with two flags attached to it. The stick represents the triple bond, and the flags are the substituents. You can’t rotate the stick to make the flags point in different directions. It’s locked in a linear arrangement, and there’s no way to have them on the “same side” or “opposite sides” of the stick.
In essence, the linearity of the triple bond eliminates the possibility of different spatial arrangements of substituents, ruling out the existence of cis-trans isomers.
Why do alkanes and alkynes not show cis-trans isomerism?
Firstly, cis-trans isomerism arises from the restricted rotation around a double bond or in a cyclic structure. This restriction prevents the groups attached to the double bond from freely rotating, leading to different spatial arrangements.
Alkanes, on the other hand, only have single bonds between their carbon atoms. These single bonds are formed by sp3 hybridized orbitals, which allow for free rotation around the bond axis. This free rotation means that the groups attached to the carbon atoms can rotate freely, making it impossible to distinguish between different spatial arrangements.
Alkynes possess a triple bond between their carbon atoms. This triple bond comprises one sigma bond and two pi bonds. While the sigma bond is formed by sp hybridized orbitals and allows for rotation, the two pi bonds are formed by the overlap of p orbitals and create a rigid, linear structure. This linearity prevents any rotation around the triple bond, but it also makes cis-trans isomerism impossible because there are only two positions for substituents, directly on either side of the triple bond.
Think of it this way: imagine you have two groups attached to a single bond. You can easily twist the bond and rotate the groups freely. Now, picture those two groups attached to a double bond. They’re stuck in a fixed position because the double bond restricts rotation. In alkanes, the single bonds allow for free rotation, making it impossible to have different spatial arrangements. In alkynes, the triple bond is linear, limiting the arrangement of groups and preventing the possibility of cis-trans isomerism.
Which isomers can show cis-trans isomerism?
In the case of cis-trans isomers, the double bond acts like the seesaw pivot. If the two groups on each carbon are on the same side of the double bond, we call it a cis isomer. If they’re on opposite sides, it’s a trans isomer.
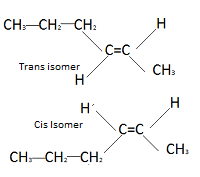
Now, let’s get back to 2-chlorobut-2-ene. This molecule has a double bond between the second and third carbon atoms, and each of these carbons has a chlorine atom and a methyl group attached. In this case, the two groups are different, which allows for cis-trans isomerism.
Think of it this way: Imagine you have a seesaw where one side has a chlorine atom and the other has a methyl group. If both the chlorine and methyl groups are on the same side of the seesaw (same side of the double bond), you’ve got the cis isomer. If they’re on opposite sides, you have the trans isomer.
To summarize, only compounds with a double bond and two different groups attached to each carbon involved in the double bond can exhibit cis-trans isomerism. 2-chlorobut-2-ene fits this description perfectly, and that’s why it can exist in both cis and trans forms.
Is cis-trans isomerism possible in alkenes?
Alkenes with the structure R–CH=CH–R can exist as cis and trans isomers. In cis isomers, the two R groups are on the same side of the carbon-to-carbon double bond. In trans isomers, the two R groups are on opposite sides of the carbon-to-carbon double bond.
Think of it like this: imagine the carbon-to-carbon double bond as a rigid bar, and the R groups are attached to it. If the R groups are both on the same side of the bar, you have a cis isomer. If they are on opposite sides, you have a trans isomer.
Cis-trans isomerism arises because of the restricted rotation around the carbon-to-carbon double bond. This restricted rotation is due to the presence of a pi bond in addition to the sigma bond that holds the two carbons together.
Now, let’s break down the reasons why this isomerism happens in alkenes:
The Double Bond: The double bond in alkenes is a crucial player. It prevents free rotation around the carbon-to-carbon bond, leading to different spatial arrangements of the R groups.
The Pi Bond: This pi bond is formed by the overlap of p orbitals above and below the plane of the sigma bond. This overlap creates a region of electron density that prevents rotation.
The R Groups: The R groups can be different or the same. The key is that they must be different from hydrogen for cis-trans isomerism to occur. If both R groups are hydrogen, you don’t have distinct isomers.
Think of it like two people holding hands. If they are facing each other, they represent a cis configuration. If they are facing opposite directions, they are in a trans configuration.
It’s important to remember that cis-trans isomerism only applies to alkenes with two different R groups attached to the double bond. If one or both R groups are hydrogen, then the isomerism doesn’t apply.
Do triple bonds have cis-trans isomers?
Triple bonds are formed by the overlap of sp hybridized orbitals. This hybridization leads to a linear geometry around the carbon atom involved in the triple bond. Imagine a straight line—that’s how the atoms are arranged.
In cis-trans isomerism, we need the possibility of different spatial arrangements around a double bond. Think of a double bond as a flat plane. On this plane, the attached groups can be on the same side (cis) or opposite sides (trans). This is simply not possible with a triple bond due to its linear structure.
Think about it this way: with a triple bond, there’s no “same side” or “opposite side” because the arrangement is a straight line. It’s like a single road with no branching lanes. There’s only one way to go.
To illustrate, consider the molecule acetylene, which has a triple bond between two carbon atoms. The two hydrogen atoms attached to the carbons are positioned at 180 degrees to each other. There is no other possible arrangement.
In contrast, consider ethene with a double bond. The two hydrogen atoms attached to the carbon atoms can be on the same side of the double bond (cis-ethene) or on opposite sides (trans-ethene).
Therefore, while cis-trans isomerism is a feature of molecules with double bonds, it is not observed in molecules with triple bonds due to their linear geometry.
Is halogenation of alkynes cis or trans?
Alkynes react with halogens like bromine or chlorine in a two-step process. First, one equivalent of the halogen adds to the alkyne, forming a trans-dihaloalkene as the main product. You’ll also get a tiny amount of the cis isomer. The second halogen molecule then adds to the trans-dihaloalkene to form a tetrahalide.
Let’s dig a little deeper into why the first addition is predominantly trans. The reaction happens through a cyclic halonium ion intermediate. This intermediate is formed when the halogen attacks the alkyne, and the positive charge on the halogen is shared with the carbon atoms.
Think of it this way: the halogen attacks one of the carbon atoms in the alkyne, and then the other carbon atom gets involved. This forms a ring-like structure. Because of the way this ring forms, the halogen atoms end up on opposite sides of the double bond in the trans-dihaloalkene.
The cis isomer forms to a much smaller extent because it requires the halogen atoms to attack from the same side of the molecule, which is less likely. It’s like trying to squeeze two big molecules into a small space – it’s just not as comfortable!
The second addition step is less picky about the stereochemistry because the trans-dihaloalkene is a much more stable molecule than the cis isomer. The halogen atoms are nicely situated on opposite sides of the double bond, and there’s no real reason for them to flip over to the same side. This makes it much easier for the second halogen molecule to attack and form the tetrahalide.
So, in summary, the trans addition is the main product in the first step of halogenation because of the cyclic halonium ion intermediate formation. The cis isomer forms a little bit, but it’s not as favored. The second step is less picky about stereochemistry because the trans-dihaloalkene is more stable and makes it easier for the second halogen molecule to attack.
Which compound does not show cis-trans isomerism?
To understand this, we need to remember the key requirements for a compound to display cis-trans isomerism. First, the molecule needs to have a carbon-carbon double bond. This restricts rotation around that bond, locking the groups on either side into specific positions. Second, each carbon involved in the double bond must have two different groups attached to it.
Now, let’s look at 1-hexene. Its structure is:
“`
CH3-CH2-CH2-CH2-CH=CH2
“`
Notice that the second carbon in the double bond (the one on the right side of the = sign) only has one hydrogen atom attached to it. The other group is a simple hydrogen. Since it has two identical groups (hydrogen), it can’t exhibit cis-trans isomerism.
Think of it this way: Imagine you have two chairs, one facing north and one facing south, but they both have the same person sitting in them. You can’t really distinguish between them, right? It’s the same principle with 1-hexene; there’s no way to create two distinct configurations because the groups on one side of the double bond are identical.
In contrast, let’s take a look at 2-butene. Its structure is:
“`
CH3-CH=CH-CH3
“`
Here, each carbon in the double bond has two different groups attached: a methyl group (CH3) and a hydrogen atom. This allows for cis-trans isomerism.
Cis isomers have similar groups on the same side of the double bond, while trans isomers have them on opposite sides.
You’re right again – cis-trans isomers often have distinct physical properties. Trans isomers tend to pack more efficiently in a crystal lattice, leading to higher melting points. Cis isomers, with their more crowded geometry, often have higher dipole moments, making them slightly more polar.
Let’s summarize: for a compound to exhibit cis-trans isomerism, it needs a carbon-carbon double bond, and each carbon in the double bond must have two different groups attached. 1-hexene fails to meet this second requirement, which is why it doesn’t display cis-trans isomerism.
What is the difference between cis and trans alkynes?
Cis and trans describe the relative positions of substituents on a double bond or a ring structure. In cis isomers, these substituents are on the same side of the double bond or ring. In trans isomers, the substituents are on opposite sides.
Think of it like this: Imagine a straight road with two lanes. If the substituents are on the same side of the road, they’re cis. If they’re on opposite sides of the road, they’re trans.
Now, let’s talk about alkynes. Alkynes are hydrocarbons that contain a triple bond. Since alkynes have a linear geometry, there’s no way to have cis and trans isomers. Why? Because all the atoms are in a straight line, there is no “same side” or “opposite side.”
To summarize, cis and trans isomers are used to describe the relative positions of substituents on double bonds or rings, but not triple bonds. Alkynes, which contain triple bonds, do not exhibit cis or trans isomerism.
See more here: Why Do Alkanes And Alkynes Not Show Cis-Trans Isomerism? | Alkynes Can Show Cis Trans Isomerism
See more new information: musicbykatie.com
Alkynes Can Show Cis-Trans Isomerism: A Surprising Twist
First, let’s define what cis-trans isomerism actually is. It’s a type of stereoisomerism where molecules have the same atoms and bonds, but they’re arranged differently in space. Think of it like this: you can have two Lego structures made of the same pieces, but they look different because the pieces are put together differently.
Now, let’s talk about alkynes. These are hydrocarbons with a triple bond between two carbon atoms. That triple bond is super strong and rigid, and it’s the key to understanding why alkynes can’t show cis-trans isomerism.
Here’s the thing: cis-trans isomerism requires the presence of a double bond in the molecule. That double bond has to be within a part of the molecule that can rotate, and that’s where the cis and trans arrangements come into play.
Imagine you have a double bond between two carbons. Each carbon has two other groups attached to it. If those groups are on the *same* side of the double bond, we call that cis. If they’re on *opposite* sides of the double bond, that’s trans.
But alkynes don’t have a double bond, they have a triple bond. That triple bond is super rigid, and it doesn’t allow any rotation. So, even if you have two different groups attached to each carbon in the alkyne, they can’t switch places to form different stereoisomers.
Think of it like a locked door. You can’t rotate the door handle because the lock is holding it in place. Similarly, the triple bond in an alkyne acts like a lock, preventing any rotation and preventing cis-trans isomerism.
Here’s an analogy that might help: imagine you have two pieces of string. One string has a single knot, and the other string has a double knot. You can easily twist and turn the string with the single knot. However, the string with the double knot is much more rigid and difficult to twist. The triple bond in an alkyne is like that double knot, making it impossible for the molecule to rotate and form different stereoisomers.
So, to answer your question directly, alkynes cannot show cis-trans isomerism. The reason is that their triple bond is too rigid to allow for the rotation needed for different spatial arrangements.
If you’re interested in learning more about stereoisomerism and the different types of isomers, I’d encourage you to do some research. There’s a whole world of fascinating chemistry out there!
FAQs
1. Why do alkenes show cis-trans isomerism but alkynes don’t?
Alkenes have a double bond, which allows for limited rotation. This rotation allows for different spatial arrangements of groups attached to the double bond, leading to cis-trans isomerism. However, alkynes have a triple bond, which is much more rigid and doesn’t allow for any rotation. Therefore, alkynes can’t exhibit cis-trans isomerism.
2. Can alkynes show any kind of isomerism?
Yes, alkynes can show other types of isomerism, such as positional isomerism and chain isomerism.
3. What is the difference between cis and trans isomers?
Cis isomers have the same groups attached to the double bond on the *same* side of the molecule. Trans isomers have the same groups attached to the double bond on *opposite* sides of the molecule.
4. What are some examples of cis and trans isomers?
Cis-2-butene and trans-2-butene are examples of cis-trans isomers. Both molecules have the same chemical formula (C4H8), but they have different spatial arrangements due to the presence of a double bond.
5. How do I determine if a molecule can show cis-trans isomerism?
To determine if a molecule can show cis-trans isomerism, look for the presence of a double bond. If the double bond is present and there are two different groups attached to each carbon of the double bond, then the molecule can show cis-trans isomerism.
I hope this explanation helps! If you have any other questions, feel free to ask.
Cis–trans isomerism | Alkenes and Alkynes | Organic chemistry
Start practicing—and saving your progress—now! https://www.khanacademy.org/science/o… Cis–trans notation can be used to describe the configuration of a double bond with exactly two … YouTube
Cis–trans isomerism (video) | Khan Academy
Cis–trans notation can be used to describe the configuration of a double bond with exactly two substituents and two hydrogens. If the two substituents are on the same side of the double bond, the configuration of the bond is cis . Khan Academy
13.2: Cis-Trans Isomers (Geometric Isomers) – Chemistry LibreTexts
Recognize that alkenes that can exist as cis-trans isomers. Classify isomers as cis or trans. Draw structures for cis-trans isomers given their names. Chemistry LibreTexts
7.5: Cis-Trans Isomerism in Alkenes – Chemistry
In one, the two chlorine atoms are locked on opposite sides of the double bond. This is known as the trans isomer. (trans: from latin meaning “across” – as in transatlantic). In the other, the two chlorine atoms are Chemistry LibreTexts
CHAPTER FOUR Alkenes and Alkynes – chemistry.sdsu.edu
Cis‐TransIsomerism – Vitamin A has five C‐C double bonds, four of which can show cis‐trans isomerism. – Vitamin A is the all‐trans isomer. Physical Properties • Alkenes sdsu.edu
Cis–trans isomerism – Wikipedia
As with organic compounds, the cis isomer is generally the more reactive of the two, being the only isomer that can reduce alkenes and alkynes to alkanes, but for a different Wikipedia
13.3: The Structure of Alkenes- Cis-Trans Isomerism
Recognize that alkenes that can exist as cis-trans isomers. Classify isomers as cis or trans. Draw structures for cis-trans isomers given their names. Chemistry LibreTexts
7.4 Cis–Trans Isomerism in Alkenes – Organic Chemistry
The cis isomer has the two methyl groups on the same side of the double bond, and the trans isomer has methyl groups on opposite sides. Cis–trans isomerism is not limited OpenStax
Chapter 5: Alkenes and Alkynes – Michigan State
These differences make it possible to separate E and Z isomers (and cis/trans since they are just a special case of E/Z) from one another. Stability of alkenes: Elimination reactions that produce alkenes tend to Open Textbook Publishing
cis-trans and E-Z naming scheme for alkenes – Khan Academy
Cis and Trans isomerism only happens around an alkene because they’re locked into the positions thanks to the pi structure. You don’t get any rotation around a pi bond whereas you do around a sigma bond so theres no cis and trans for alkanes Khan Academy
Cis–Trans Isomerism | Alkenes And Alkynes | Organic Chemistry | Khan Academy
Cis And Trans Isomers
13A: Classifying Alkenes As Cis Or Trans
Using Cis/Trans Versus E/Z To Describe Double Bonds
Cis-Trans And E-Z Naming Scheme For Alkenes | Alkenes And Alkynes | Organic Chemistry | Khan Academy
13: Identifying Cis And Trans Isomers
Naming Alkenes Using E Z System – Iupac Nomenclature
Ep.13 เคมีอินทรีย์ Cis-Trans E-Z Isomer
Link to this article: alkynes can show cis trans isomerism.
See more articles in the same category here: https://musicbykatie.com/wiki-how/
