Table of Contents
What happens when alcohol reacts with SOCl2?
You might be wondering why SOCl2 (thionyl chloride) is such a good reagent for turning alcohols into alkyl chlorides. It’s all about the byproducts! The reaction produces SO2 (sulfur dioxide) and HCl, both gases. These gases escape, driving the reaction forward and ensuring a good yield of your desired alkyl chloride.
Think of it like this: Imagine you’re baking a cake and you want to get rid of excess steam. The steam is like the SO2 and HCl in this reaction. By letting the steam escape, you create space for more cake to bake, just like how the gases escaping in the reaction allow more alcohol to react with SOCl2 to form the alkyl chloride.
Let’s break down the reaction a little more:
The Alcohol: The oxygen atom in the alcohol molecule is a bit lonely. It wants to bond with something else.
The SOCl2: The sulfur atom in SOCl2 is looking for a new friend too.
The Matchmaker: The sulfur atom in SOCl2 is actually quite a good matchmaker. It helps the oxygen atom in the alcohol connect with one of the chlorine atoms from SOCl2. This kicks off the reaction.
The Byproducts: The sulfur atom in SOCl2 doesn’t want to be left out! It grabs the remaining oxygen from the alcohol and forms SO2. The extra chlorine atom from SOCl2 combines with a hydrogen from the alcohol to create HCl. Since SO2 and HCl are gases, they quickly escape, leaving the alkyl chloride behind.
So, in a nutshell, SOCl2 is a great choice for making alkyl chlorides because it produces gaseous byproducts that leave the reaction mixture, allowing the reaction to proceed smoothly and efficiently. This makes it a popular reagent in organic chemistry labs.
Is SOCl2 SN1 or SN2?
SOCl2 is a powerful reagent that reacts with alcohols to form alkyl chlorides. This reaction typically proceeds via an SN2 mechanism. SN2 stands for bimolecular nucleophilic substitution. In this case, the chloride ion acts as a nucleophile, attacking the carbon atom attached to the hydroxyl group. The OH group leaves as a good leaving group, leading to the formation of an alkyl chloride. This mechanism is favored when the alcohol is primary or secondary, meaning the carbon attached to the OH group has one or two other carbon atoms attached to it.
COCl2, on the other hand, tends to favor the SN1 mechanism. SN1 stands for unimolecular nucleophilic substitution. In this mechanism, the OH group leaves first to form a carbocation intermediate. This intermediate is then attacked by a chloride ion. This mechanism is favored when the alcohol is tertiary, meaning the carbon attached to the OH group has three other carbon atoms attached to it.
Let me explain why SOCl2 prefers SN2 and COCl2 prefers SN1 by taking a look at their reaction mechanisms.
SOCl2 Reaction with Alcohols
The reaction of SOCl2 with an alcohol proceeds in a two-step process. First, the alcohol reacts with SOCl2 to form a chlorosulfite intermediate. This intermediate is then attacked by a chloride ion, leading to the formation of an alkyl chloride and sulfur dioxide.
“`
ROH + SOCl2 → ROSOCl + HCl
ROSOCl + Cl- → RCl + SO2
“`
COCl2 Reaction with Alcohols
The reaction of COCl2 with an alcohol also proceeds in a two-step process. The first step involves the formation of an alkyl chlorocarbonate intermediate. This intermediate is then attacked by a chloride ion, leading to the formation of an alkyl chloride and carbon dioxide.
“`
ROH + COCl2 → ROCOCl + HCl
ROCOCl + Cl- → RCl + CO2
“`
Which Mechanism is Favored?
The mechanism of the reaction is determined by the structure of the alcohol. If the alcohol is primary or secondary, the SN2 mechanism is favored because the carbon atom attached to the OH group is less hindered. This allows the chloride ion to attack the carbon atom from the back side, leading to inversion of configuration.
If the alcohol is tertiary, the SN1 mechanism is favored because the carbon atom attached to the OH group is highly hindered. This prevents the chloride ion from attacking the carbon atom from the back side. Instead, the OH group leaves first to form a carbocation intermediate, which is then attacked by the chloride ion.
Conclusion
In summary, SOCl2 and COCl2 are both useful reagents for converting alcohols into alkyl chlorides. However, the mechanism of the reaction can vary depending on the structure of the alcohol. SOCl2 generally favors SN2 mechanisms, while COCl2 favors SN1 mechanisms. This difference is due to the different reactivities of the two reagents and the different stabilities of the intermediates formed.
I hope this helps! If you have any other questions, feel free to ask. I’m here to help you understand the world of chemistry.
What is the mechanism of alcohol and thionyl chloride?
First, the alcohol’s oxygen atom, with its lone pairs, acts as a nucleophile. It attacks the sulfur atom of thionyl chloride, kicking off a chloride ion. This creates an intermediate, known as a protonated alkyl chlorosulfite. This intermediate is short-lived but crucial to the reaction.
Next, a base (often chloride ions or the alcohol itself) removes a proton from the intermediate, leading to the formation of the alkyl chlorosulfite. This is an inorganic ester, a key compound in the reaction.
The beauty of this reaction is that it results in the replacement of the hydroxyl group (-OH) on the alcohol with a chlorine atom (-Cl), forming an alkyl chloride. This transformation is highly useful in organic synthesis.
To better understand the process, visualize the thionyl chloride molecule (SOCl2). The sulfur atom has a partial positive charge, making it attractive to the electron-rich oxygen of the alcohol. The lone pairs on the alcohol’s oxygen attack the sulfur, forming a bond. The sulfur-oxygen bond in thionyl chloride is weak and easily breaks, allowing the chloride ion to depart.
Now, picture the newly formed intermediate. It has a proton attached to the oxygen atom that was originally part of the alcohol. A base then comes in and plucks this proton, creating the alkyl chlorosulfite. This molecule has a sulfur atom bonded to both an alkyl group and a chlorine atom.
The whole reaction is a beautiful example of how electron-rich and electron-poor species interact. The nucleophilic attack, the formation of the intermediate, and the deprotonation all contribute to this fundamental organic reaction that plays a vital role in organic synthesis.
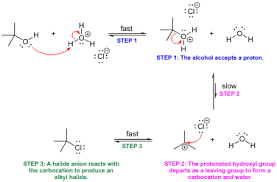
Do alcohols undergo SN1 or SN2?
The acidity of an alcohol is determined by two main factors: resonance and steric bulk. Resonance helps to stabilize the lone pair on the oxygen atom, making the alcohol more acidic. On the other hand, steric bulk, which refers to the size of the groups attached to the carbon atom bearing the hydroxyl group (OH), can hinder the formation of the conjugate base, making the alcohol less acidic.
Now, you’re probably wondering how acidity plays a role in SN1 and SN2 reactions. Here’s the crucial point: alcohols can only undergo SN1 or SN2 reactions after they’ve been protonated.
Why?
Think of it this way: Protonation converts the alcohol’s -OH group into a good leaving group, water (H2O). This is because water is a much more stable molecule compared to the hydroxide ion (OH-) present in the original alcohol.
Let’s break it down with an example:
Consider the reaction of ethanol (CH3CH2OH) with a strong acid like hydrochloric acid (HCl).
Step 1: Protonation
* The proton from the acid (H+) will attack the lone pair on the oxygen atom of the ethanol, forming ethoxonium ion (CH3CH2OH2+).
Step 2: Leaving group departure
* The ethoxonium ion is now a much better leaving group than the original ethanol molecule. It readily loses a water molecule (H2O), leaving behind a carbocation (CH3CH2+).
Step 3: Nucleophilic attack
* Now, the carbocation is susceptible to attack by a nucleophile. Depending on the conditions and the nature of the nucleophile, the carbocation can participate in either an SN1 or SN2 reaction.
So, in essence, the protonation of alcohols sets the stage for SN1 or SN2 reactions by creating a good leaving group.
To summarize, the acidity of an alcohol plays a crucial role in determining its reactivity in SN1 and SN2 reactions. It is the protonated form of the alcohol that is capable of undergoing these reactions, due to the formation of a good leaving group.
Is SOCl2 a nucleophile or electrophile?
Let’s break down why SOCl2 acts as an electrophile. It’s all about the structure and the way the electrons are distributed. Sulfur, being in the center, is more electronegative than chlorine. This means sulfur pulls electron density away from the chlorine atoms, making the chlorine atoms slightly electron-deficient.
Now, the chlorine atoms are attached to sulfur through a covalent bond. However, the electron density is shifted towards sulfur, making the chlorine atoms more susceptible to attack by electron-rich species. This is precisely what makes SOCl2 a good electrophile. It readily accepts electrons from nucleophiles, leading to reactions where the chlorine atoms are displaced.
A classic example is the reaction of SOCl2 with alcohols to form alkyl chlorides. The oxygen atom in the alcohol, being electron-rich, acts as a nucleophile and attacks the electron-deficient chlorine atom in SOCl2. This leads to the displacement of chlorine and the formation of an alkyl chloride.
So, while SOCl2 itself is an electrophile, the chlorine atoms it carries have a strong tendency to act as nucleophiles in reactions. This dual nature makes SOCl2 a versatile reagent, playing a crucial role in various organic reactions.
Is SOCl2 retention or inversion?
The truth is, SOCl2’s effect on configuration depends heavily on the solvent you use. You can get straight inversion with the right solvent, or a mix of retention and inversion with others.
Let’s dive a little deeper into why this happens.
Think of SOCl2 as a bit of a chameleon. It can change its behavior based on its environment. In some solvents, SOCl2 acts as a straightforward inverting agent. This means it flips the configuration of the molecule you’re reacting with. It’s like switching the left and right sides of a mirror image.
But in other solvents, things get a little more complicated. SOCl2 can actually react with the solvent itself, forming a new intermediate compound. This intermediate can then react with your molecule, leading to both inversion and retention of configuration. It’s like having a coin flip—sometimes you get heads (inversion), sometimes you get tails (retention), and sometimes you even get both.
So, the next time you encounter SOCl2, remember to consider the solvent. It’s the key to understanding whether you’ll get inversion, retention, or a mix of both.
Is racemisation in SN1 or SN2?
SN1 reactions involve a carbocation intermediate. This intermediate is sp2 hybridized, which means it’s planar and has a trigonal planar geometry. This geometry allows for equal probability of attack from either side by the nucleophile. This is why we get a racemic mixture of products in SN1 reactions. A racemic mixture is a 50:50 mixture of enantiomers – mirror images that cannot be superimposed.
SN2 reactions are different. They are concerted reactions, meaning they happen all in one step, with no intermediates. The nucleophile attacks the substrate from the backside, causing the inversion of configuration at the chiral center. This means there’s no chance for the carbocation to form, and therefore no chance for racemization.
Think of it this way: in an SN1 reaction, the carbocation is like a piece of paper with both sides equally exposed, so a nucleophile can attack from either side. In an SN2 reaction, the nucleophile is like a ninja, sneaking in from behind and attacking the substrate in one swift move, causing it to flip its configuration.
Now, let’s get into a bit more detail. Carbocation intermediates are very unstable and are constantly in motion. They are highly reactive, so a nucleophile can attack from either side to form two different products, the enantiomers. This process is what leads to the racemic mixture of products that we see in SN1 reactions.
Remember that SN1 reactions are favored when the leaving group is a good one, such as a halide. And, since the carbocation is an unstable intermediate, it’s more likely to form if the substrate is tertiary or secondary, making it easier for the leaving group to depart.
SN2 reactions, on the other hand, prefer a primary substrate, where the nucleophile can easily attack from the backside. They are also favored by good nucleophiles, but poor leaving groups.
So, to sum it up, SN1 reactions lead to racemization because they involve carbocation intermediates. SN2 reactions don’t have carbocation intermediates and therefore don’t result in racemization. This is because the nucleophile attacks from the backside in a single step, leading to inversion of configuration.
Is SOCl2 SN2?
You’re right to wonder if the substitution step in the reaction of SOCl2 with an alcohol is SN2. The fact that the reaction leads to an inversion of configuration in the presence of pyridine strongly suggests that the substitution step indeed follows the SN2 pathway. This is similar to the reaction with PBr3, which also exhibits SN2 characteristics.
Here’s a deeper look at why we can confidently say the substitution step is SN2:
Stereochemistry: The inversion of configuration is a classic hallmark of SN2 reactions. This means that the attacking nucleophile (in this case, chloride ion from SOCl2) approaches the carbon bearing the leaving group (the hydroxyl group of the alcohol) from the backside, leading to a flipped configuration at the reaction center.
Concerted Mechanism: SN2 reactions are known for their concerted nature, meaning the bond breaking and bond formation happen simultaneously in a single step. This is supported by the observation that SOCl2 reactions are generally very fast, a characteristic of SN2 reactions.
How does SOCl2 actually react with an alcohol?
The reaction of SOCl2 with an alcohol is a multi-step process that involves several key intermediates. Let’s break it down:
1. Nucleophilic Attack: The alcohol acts as a nucleophile and attacks the sulfur atom of SOCl2, leading to the formation of an alkoxysulfonyl chloride intermediate.
2. Chlorine Leaving Group: The sulfur atom in the intermediate is electron-deficient due to the presence of the two chlorine atoms. This makes it susceptible to attack by the chloride ion, which acts as a leaving group.
3. Formation of Alkyl Chloride: The alkoxysulfonyl chloride intermediate further reacts with another chloride ion, leading to the formation of the desired alkyl chloride product.
So, while the overall reaction may seem complex, the crucial substitution step that generates the alkyl chloride product is indeed an SN2 process. The stereochemical inversion and the concerted nature of the reaction strongly support this conclusion.
This understanding is vital for predicting reaction outcomes and designing synthetic strategies.
See more here: Is Socl2 Sn1 Or Sn2? | Alcohol With Thionyl Chloride Mechanism
Can thionyl chloride convert alcohol into alkyl halides?
Let’s break down why this reaction is so useful. Thionyl chloride reacts with alcohols in a process called chlorination, replacing the hydroxyl group (-OH) with a chlorine atom (-Cl). This results in the formation of an alkyl chloride along with byproducts such as sulfur dioxide (SO2) and hydrogen chloride (HCl).
The reaction is typically carried out in the presence of a base like pyridine, which helps to neutralize the HCl that is formed. Pyridine is a good choice of base because it is non-nucleophilic, meaning that it won’t react with the alkyl chloride product.
The beauty of using thionyl chloride lies in its ability to convert alcohols into alkyl chlorides in a single step, eliminating the need for multiple reaction steps. This makes it a highly efficient and time-saving method in organic synthesis.
Here’s a simple illustration of the reaction:
ROH + SOCl2 → RCl + SO2 + HCl
Where R represents an alkyl group.
Let’s look at an example. Imagine you have ethanol (CH3CH2OH). When you react it with thionyl chloride (SOCl2), you’ll get ethyl chloride (CH3CH2Cl), sulfur dioxide (SO2), and hydrogen chloride (HCl) as byproducts.
CH3CH2OH + SOCl2 → CH3CH2Cl + SO2 + HCl
This reaction is widely used in organic chemistry for synthesizing a variety of alkyl chlorides, which are valuable starting materials for various reactions, including:
Nucleophilic substitution reactions: Alkyl chlorides are excellent substrates for nucleophilic substitution reactions, allowing for the introduction of various functional groups.
Grignard reactions: Alkyl chlorides are used to prepare Grignard reagents, which are powerful tools for carbon-carbon bond formation.
Wittig reactions: Alkyl chlorides can be used in Wittig reactions to synthesize alkenes.
Overall, the reaction of alcohols with thionyl chloride is a valuable tool for organic chemists, offering a convenient and efficient route to synthesize alkyl chlorides, essential building blocks for various organic compounds.
See more new information: musicbykatie.com
Alcohol With Thionyl Chloride Mechanism | What Happens When Alcohol Reacts With Socl2?
10.9 Reactions of Alcohols with Thionyl Chloride
If you take an alcohol and add thionyl chloride, it will be converted into an alkyl chloride. The byproducts here are hydrochloric acid (\(HCl\)) and sulfur dioxide (\(SO_2\)). Note: there are significant differences in how this reaction is taught at different schools. Chemistry LibreTexts
SOCl2 Mechanism For Alcohols To Alkyl Halides: SN2
Most of the time, the reaction of alcohols with thionyl chloride is taught as an S N 2 reaction. And indeed, on primary alcohols this is definitely the case. The problem arises with secondary alcohols, where Master Organic Chemistry
17.6: Reactions of Alcohols – Chemistry LibreTexts
The mechanism for both reactions start by making the alcohol’s -OH a better leaving group through conversion to an intermediate. Thionyl chloride creates an intermediate chlorosulfite (-OSOCl 2) compound and Chemistry LibreTexts
Preparation of alkyl halides from alcohols – Khan Academy
And so if we start with this alcohol over here on the left, and we add SOCl2, which is called thionyl chloride, and pyridine to it. We’re going to substitute a chlorine atom for the OH group. And this mechanism occurs via an SN2 type mechanism, Khan Academy
Minute Mechanism – Primary Alcohol to Chloride (SOCl2) – YouTube
The Primary Alcohol to Chloride using Thionyl Chloride Mechanism / chemcomplete Support the Channel! Buy Walkthrough Guides Here: https://www.chemcomplete.com/buy-guides Visit us Online:… YouTube
Reactions of Alcohols – Rutgers University
Ch11 Reacns of Alcohols (landscape).docx Page 14 Thionyl Chloride Thionyl chloride (SOCl 2) is the usual method of choice for preparing alkyl chlorides from alcohols. The crab.rutgers.edu ARCHIVE
Thionyl Chloride (SOCl2) – Master Organic Chemistry
Thionyl chloride (SOCl 2) is a useful reagent for converting alcohols to alkyl halides and for converting carboxylic acids to acid chlorides. We’ve met it previously in the chapter on alcohols. ( See Master Organic Chemistry
Alcohols to Alkyl Halides: Reagents, Mechanism, and … – JoVE
Stereochemistry. Most importantly, the choice of reagent influences the stereochemistry of the product formed. The use of thionyl chloride leads to an inversion of configuration, JoVE
10.5 Preparing Alkyl Halides from Alcohols – OpenStax
Primary and secondary alcohols are best converted into alkyl halides by treatment with either thionyl chloride (SOCl 2) or phosphorus tribromide (PBr 3). These reactions, OpenStax
Mechanism Of Alcohol With Thionyl Chloride
Reaction Of Alcohols With Thionyl Chloride In Pyridine Darzen’S Process By Dr. Manu Kaushal
Alcohol Reactions – Hbr, Pbr3, Socl2
Chem 222: Reaction Of Alcohols With Thionyl Chloride
Alcohol To Alkyl Chloride With Socl2 Mechanism | Organic Chemistry
Reaction Of Alcohols With Thionyl Chloride
Minute Mechanism – Primary Alcohol To Chloride (Socl2)
Mechanism Of Primary Alcohol To Primary Alkyl Halide Using Thionyl Chloride (Socl2) 001
Link to this article: alcohol with thionyl chloride mechanism.
See more articles in the same category here: https://musicbykatie.com/wiki-how/
