Are you looking for an answer to the topic “Does K3PO4 dissociate in water?“? We answer all your questions at the website Musicbykatie.com in category: Digital Marketing Blogs You Need To Bookmark. You will find the answer right below.
Answer and Explanation: Potassium phosphate is considered to be soluble in water.K3PO4(aq). Balanced chemical reaction (dissociation): K₃PO₄(aq) → 3K⁺(aq) + PO₄³⁻(aq). K₃PO₄ is potassium phosphate, a water-soluble ionic salt. In water potassium phosphate, ionic compound, dissociates on positive potassium ion (cations) and negative phosphate ions (anions).Potassium phosphate tribasic – is a water-soluble ionic salt which has the chemical formula K3PO4.
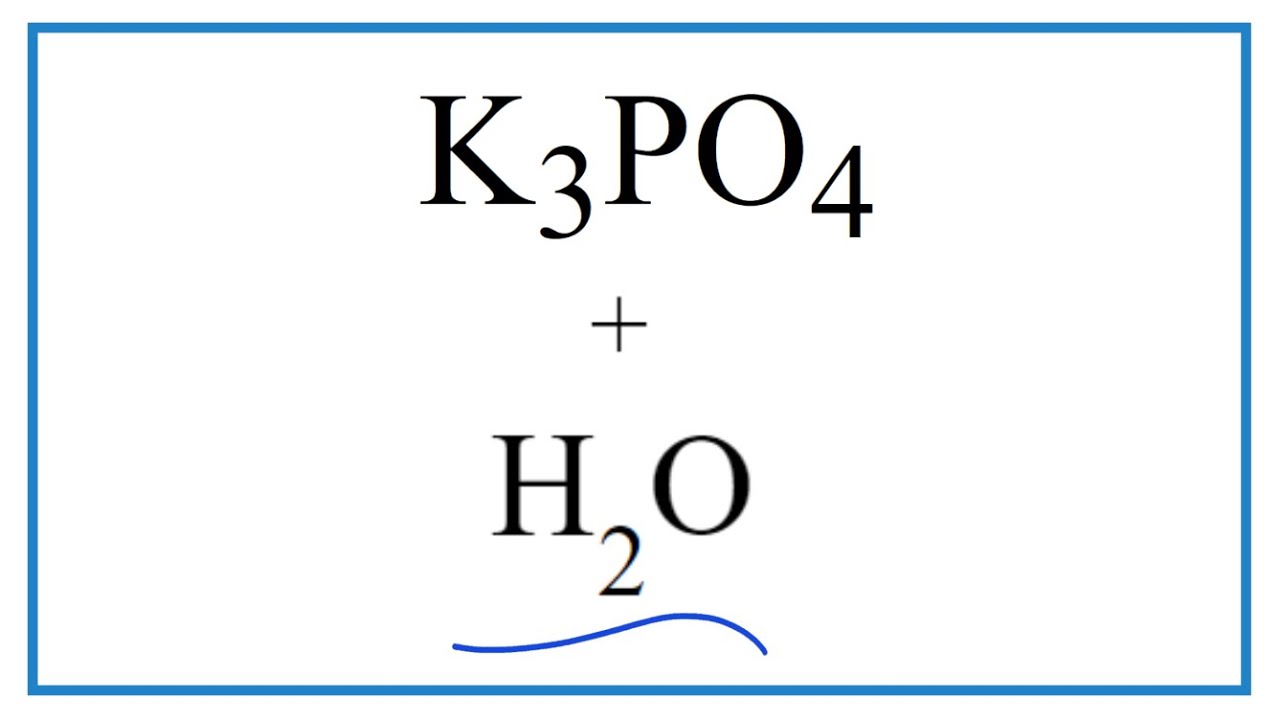
Table of Contents
Can K3PO4 dissolve in water?
Answer and Explanation: Potassium phosphate is considered to be soluble in water.
Does K3PO4 dissociate?
K3PO4(aq). Balanced chemical reaction (dissociation): K₃PO₄(aq) → 3K⁺(aq) + PO₄³⁻(aq). K₃PO₄ is potassium phosphate, a water-soluble ionic salt. In water potassium phosphate, ionic compound, dissociates on positive potassium ion (cations) and negative phosphate ions (anions).
Equation for K3PO4 + H2O (Potassium phosphate + Water)
Images related to the topicEquation for K3PO4 + H2O (Potassium phosphate + Water)

Is K3PO4 a soluble or insoluble?
Potassium phosphate tribasic – is a water-soluble ionic salt which has the chemical formula K3PO4.
Is sodium phosphate soluble in water?
Is Na3PO4 (Sodium phosphate) soluble or insoluble in water? The answer is that it is soluble in water.
What are the ions in K3PO4?
This means that in each formula unit there are 3 potassium ions and 1 phosphate ion.
What state of matter is K3PO4?
Tripotassium phosphate, also called tribasic potassium phosphate is a water-soluble salt with the chemical formula K3PO4(H2O)x (x = 0, 3, 7, 9). Tripotassium phosphate is basic.
Is potassium phosphate a strong electrolyte?
The compound potassium phosphate is a strong electrolyte.
See some more details on the topic Does K3PO4 dissociate in water? here:
Answer in General Chemistry for jimbo #92002 – Assignment …
K3PO4(aq). … Balanced chemical reaction (dissociation): K₃PO₄(aq) → 3K⁺(aq) + PO₄³⁻(aq). K₃PO₄ is potassium phosphate, a water-soluble ionic …
Complete this equation for the dissociation of K3PO4(aq …
Balanced chemical reaction (dissociation): K₃PO₄(aq) → 3K⁺(aq) + PO₄³⁻(aq). K₃PO₄ is potassium phosphate, a water-soluble ionic salt.
chem exam 2 Flashcards – Quizlet
In solution, K3PO4 dissociates into K+ and ions and Ca(NO3)2 dissociates into Ca2+ and ions. According to Table 4.2, calcium ions (Ca2+) and phosphate ions ( ) …
Is potassium phosphate a solid liquid or gas?
Physical properties: Potassium phosphate is a white, odorless and deliquescent powder. The density of this salt is 2.33 g/L. Its melting point is 252.6 °C and the boiling point is 400 °C.
Does magnesium phosphate dissolve in water?
…
Trimagnesium phosphate.
| Names | |
|---|---|
| Molar mass | 262.855 g·mol−1 |
| Appearance | White crystalline powder |
| Melting point | 1,184 °C (2,163 °F; 1,457 K) |
| Solubility in water | Insoluble |
Is K3PO4 Soluble or Insoluble in Water?
Images related to the topicIs K3PO4 Soluble or Insoluble in Water?
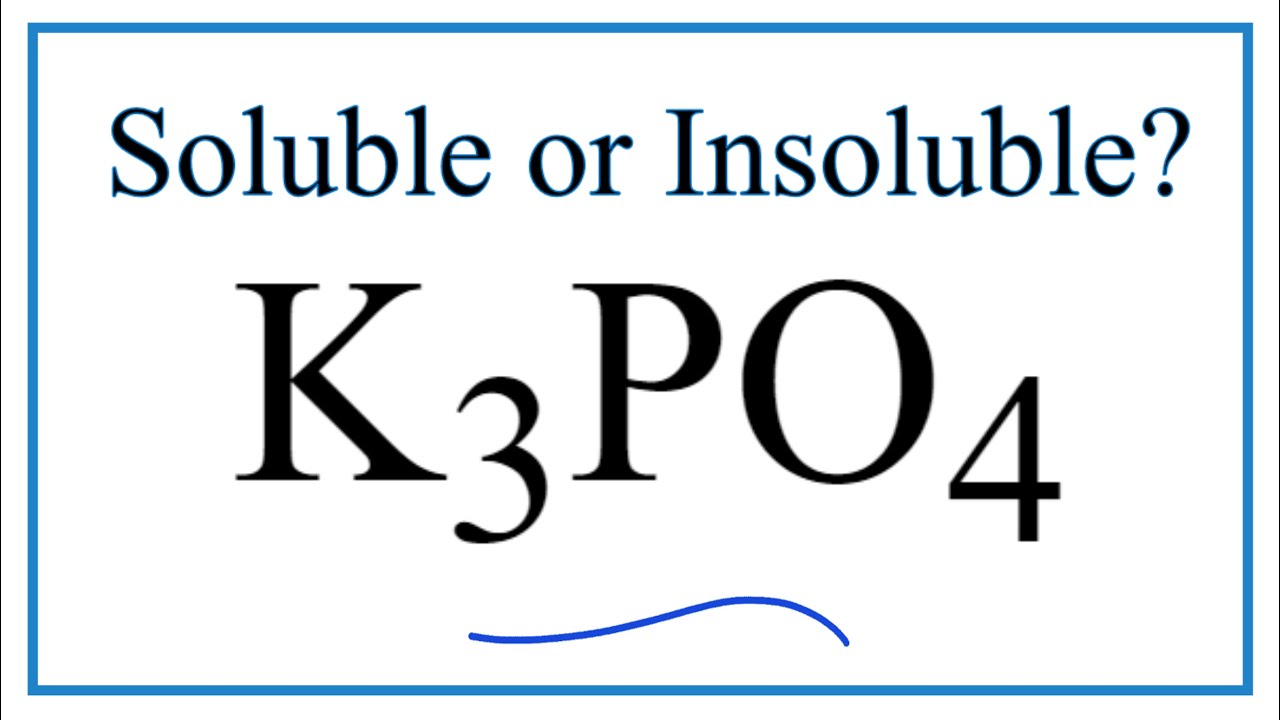
Is Iron III phosphate soluble in water?
Although it is insoluble in water, it is soluble to varying degrees in dilute hydrochloric-acid solutions, such as those in the stomach.
What is the name of K3PO4?
Potassium phosphate | K3PO4 – PubChem.
Is h3po4 a strong or weak electrolyte?
Weak Electrolyte Examples
HC2H3O2 (acetic acid), H2CO3 (carbonic acid), NH3 (ammonia), and H3PO4 (phosphoric acid) are all examples of weak electrolytes. Weak acids and weak bases are weak electrolytes. In contrast, strong acids, strong bases, and salts are strong electrolytes.
What is potassium phosphate made of?
Potassium phosphate consists of the dietary minerals potassium and phosphorus in the form of phosphate. The U.S. Food and Drug Administration (FDA) categorizes potassium phosphate as “generally recognized as safe” (GRAS) as a food additive.
Does phosphate dissolve in water?
Many phosphates are soluble in water at standard temperature and pressure. The sodium, potassium, rubidium, caesium, and ammonium phosphates are all water-soluble. Most other phosphates are only slightly soluble or are insoluble in water.
Which phosphate salt is insoluble in water?
h) All phosphates are insoluble except those of sodium, potassium and ammonium. Some hydrogen phosphates, such as Ca(H2PO4)2, are soluble. I) All sulfides are insoluble except those of ammonium, sodium, calcium, potassium, magnesium, barium and strontium. All of these are slightly hydrolyzed in water.
How do you dissolve sodium phosphate?
Prepare sodium phosphate dibasic stock (0.5 M) by dissolving 35.5 g of sodium phosphate dibasic in a final volume of 500 mL of H2O. Some crystallization will occur when the solution is stored at 4ºC. Warm on a hot plate and stir until the crystals dissolve.
How many potassium ions are in K3PO4?
In order to form a neutral compound, 3 potassium ions are needed to satisfy the 3- charge on the phosphate ion.
Is K3PO4 acidic, basic, or neutral (dissolved in water)?
Images related to the topicIs K3PO4 acidic, basic, or neutral (dissolved in water)?

What is the charge of the potassium ion in K3PO4?
| Property | Value | Source |
|---|---|---|
| Physiological Charge | -2 | ChemAxon |
| Hydrogen Acceptor Count | 4 | ChemAxon |
| Hydrogen Donor Count | 0 | ChemAxon |
| Polar Surface Area | 86.25 Ų | ChemAxon |
Is K3PO4 a normal salt?
Potassium phosphate (K3PO4) solution is basic salt since it is made by the reaction of a strong base potassium hydroxide (KOH ) and weak acid phosphoric acid(H3PO4) thus aqueous solution of Potassium phosphate is basic .
Related searches to Does K3PO4 dissociate in water?
- k3po4 molar mass
- complete the equation for the dissociation of k3po4aq
- does k3po4 dissolve in water
- k3po4 balanced equation
- k3po4 compound name
- k3po4 formula
- k3po4 state of matter
- k3po4 ions
- k3po4 positive and negative ion
Information related to the topic Does K3PO4 dissociate in water?
Here are the search results of the thread Does K3PO4 dissociate in water? from Bing. You can read more if you want.
You have just come across an article on the topic Does K3PO4 dissociate in water?. If you found this article useful, please share it. Thank you very much.
