Are you looking for an answer to the topic “Does Aluminium have a low heat capacity?“? We answer all your questions at the website Musicbykatie.com in category: Digital Marketing Blogs You Need To Bookmark. You will find the answer right below.
Now to aluminum. Its heat capacity is not all that low. It is way higher than those of titanium, iron, copper, not to mention the heavier metals. It is, however, low when compared to water.The aluminum’s temperature changed less than the copper’s did under the same conditions. Thus, the aluminum must require more energy to change its temperature. Therefore, aluminum has the higher specific heat.The heavier elements contain fewer atoms to absorb the energy per gram of material and therefore tend to have lower specific heat capacities.
| Substance | specific heat capacity Cp,s (J/g °C) | molar heat capacity Cp,m (J/mol °C) |
|---|---|---|
| air | 1.012 | 29.19 |
| aluminum | 0.89 | 24.2 |
| argon | 0.5203 | 20.786 |
| copper | 0.385 | 24.47 |
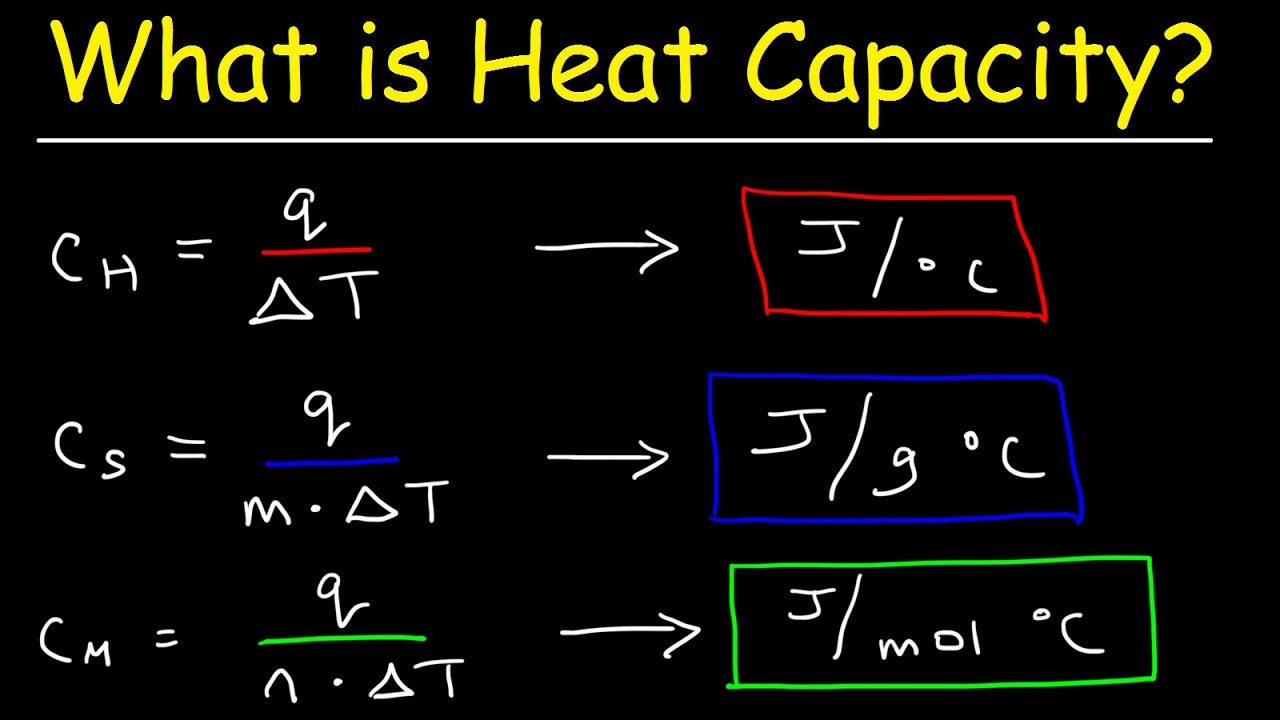
Table of Contents
Does aluminum have high or low heat capacity?
| Substance | specific heat capacity Cp,s (J/g °C) | molar heat capacity Cp,m (J/mol °C) |
|---|---|---|
| air | 1.012 | 29.19 |
| aluminum | 0.89 | 24.2 |
| argon | 0.5203 | 20.786 |
| copper | 0.385 | 24.47 |
Does aluminium have high specific heat capacity?
The aluminum’s temperature changed less than the copper’s did under the same conditions. Thus, the aluminum must require more energy to change its temperature. Therefore, aluminum has the higher specific heat.
What Is The Difference Between Specific Heat Capacity, Heat Capacity, and Molar Heat Capacity
Images related to the topicWhat Is The Difference Between Specific Heat Capacity, Heat Capacity, and Molar Heat Capacity

Why does aluminum have a low specific heat capacity?
The heavier elements contain fewer atoms to absorb the energy per gram of material and therefore tend to have lower specific heat capacities.
What material has the lowest heat capacity?
Styrofoam and many ceramics are quite heat resistant, but the best “insulator” is actually a total vacuum. Gold, being a heavy metal, has a very low heat capacity [c=0.128 J/gK]. Radon also has a very high molecular weight, and has a heat capacity of just 0.09 J/gK.
Which metal has highest heat capacity?
Thermal capacity is the amount of heat required to increase the temperature of an object by one degree. The following table provides the values of the thermal capacity of some of the metals and Aluminum. It shows that Aluminum has the highest thermal capacity.
Which metal is slowly heated?
Answer. The process of annealing can soften a variety of metals. Brass, steel, iron copper and silver can all be made weaker by heating the metal to a set temperature and cooling it slowly.
Does metal have a low heat capacity?
Metals such as iron have low specific heat. It doesn’t take much energy to raise their temperature. That’s why a metal spoon heats up quickly when placed in a cup of hot coffee. Sand also has a relatively low specific heat.
See some more details on the topic Does Aluminium have a low heat capacity? here:
Which metal heats up fastest, Aluminum, Copper, or Silver?
Specific heat capacity: Aluminum 0.91 J/g°C Copper 0.39 J/g°C Silver 0.240 … or molecules having higher energy), the atoms of the metal will gain more …
Why Doesn’t Aluminum Foil Feel Hot After It’s Taken Out Of An …
Aluminum foil has a low thermal mass on account of having such low mass and such a high surface area. That’s why …
Properties and Characteristics of Aluminum and Aluminum …
1.3 Physical Properties of Aluminum Alloys Those physical properties include (Ref 1.4–1.7): The specific heat capacity of aluminum alloys (816 to 1050 /kg K, …
UCSB Science Line
So far so good, right? “OK, great, why do we care about that?” This means that even though iron has a lower specific heat capacity than aluminum …
What is considered low heat capacity?
Heat capacity is related to a substance’s ability to retain heat and the rate at which it will heat up or cool. For example, a substance with a low heat capacity, such as iron , will heat and cool quickly, while a substance with a high heat capacity, such as water , heats and cools slowly.
What is the specific heat capacity of aluminium?
The actual value for the specific heat capacity of aluminium is 900 J/kg°C.
Is aluminum a good conductor of heat?
Electrical and Thermal Conductivity
Aluminum is an excellent heat and electricity conductor and in relation to its weight is almost twice as good a conductor as copper.
Does aluminium retain heat?
Aluminium is both a great insulator of heat and a terrible insulator of heat (or a good conductor of heat).
What heats up faster aluminum or copper?
Copper: Heat Conductivity. As you can see in this table, copper is more conductive than aluminum. In fact, aluminum only has 60% of the thermal conductivity that copper does.
Working Out the Specific Heat Capacity of an Aluminium Block Science4Breakfast GCSE Science
Images related to the topicWorking Out the Specific Heat Capacity of an Aluminium Block Science4Breakfast GCSE Science

What materials have high heat capacity?
For example water and concrete have a high capacity to store heat and are referred to as ‘high thermal mass’ materials.
What is the heat capacity of metal?
| Specific Heat Capacity of Metals Table Chart | ||
|---|---|---|
| Metal | Btu/(lb-°F) | J/(g-°C) |
| Carbon Steel | 0.120 | 0.502416 |
| Cast Iron | 0.110 | 0.460548 |
| Cesium | 0.057 | 0.2386476 |
Which materials has more specific heat?
Among the given options water has highest specific heat .
How fast does aluminum heat?
The aluminum conducted heat the fastest at an average of 14 seconds. The bronze was the second fastest at 16 seconds. The silver nickel averaged 19 seconds to conduct heat and appeared to be the strongest metal used in the experiment, as it did not melt or bend.
Why does aluminum have a higher specific heat than copper?
The SHC of aluminum will be higher than iron and copper, this is because the volume the less dense the meta lis, thus the higher the SHC because the metals contains big atoms which slowly heat up thus more energy is needed to make the molecules get hot and move around.
Which is a poor conductor of heat?
Metals and stone are considered good conductors since they can speedily transfer heat, whereas materials like wood, paper, air, and cloth are poor conductors of heat.
What happens to aluminum when heated?
When the temperature of aluminium is increased, the metal expands and this is called thermal expansion.
Is aluminum a metal?
Aluminium is a silvery-white, lightweight metal. It is soft and malleable.
Which element has the highest specific heat capacity?
There is a simple much overlooked reason for hydrogen having the greatest specific heat capacity (SHC for short). Remember that specific heat capacity is the heat capacity per unit weight. Since hydrogen is the lightest element its SHC is high.
Do all metals have the same specific heat?
All metals have the same specific heat capacity.
The Specific Heat Capacity of Aluminium
Images related to the topicThe Specific Heat Capacity of Aluminium

How do you know what has a higher specific heat capacity?
Let’s take water ( c=4.184 J/g∘C ) and compare it to copper solid ( c=0.385 J/g∘C ). The difference is clear: The higher the specific heat capacity, the smaller the temperature change for the same amount of heat applied to the same mass of substance.
Do the metals have a higher or a lower specific heat than water?
The specific heat of metals are lower than that of water. Specific heat capacity is the measurement of how much energy (in J) has to be added to 1 kg of a substance to increase the temperature of that substance by 1oC .
Related searches to Does Aluminium have a low heat capacity?
- does water have a low specific heat capacity
- does aluminium have a low heat capacity effect
- does aluminum foil conduct heat
- why does aluminum have a high specific heat capacity
- what accounts for the high specific heat of water
- which metal has the lowest specific heat capacity
- how do the specific heat capacities of metals compare with those of liquids
- does aluminium have a low heat capacity of water
- heat capacity of aluminum
- does aluminum foil transfer heat through convection
Information related to the topic Does Aluminium have a low heat capacity?
Here are the search results of the thread Does Aluminium have a low heat capacity? from Bing. You can read more if you want.
You have just come across an article on the topic Does Aluminium have a low heat capacity?. If you found this article useful, please share it. Thank you very much.
