Are you looking for an answer to the topic “Do Electrons Actually Move?“? We answer all your questions at the website Musicbykatie.com in category: Digital Marketing Blogs You Need To Bookmark. You will find the answer right below.
Electrons do not move along a wire like cars on a highway. Actually, Any conductor (thing that electricity can go through) is made of atoms. Each atom has electrons in it. If you put new electrons in a conductor, they will join atoms, and each atom will deliver an electron to the next atom.With all of this in mind, an electron in a stable atomic state does not move in the sense of a solid little ball zipping around in circles like how the planets orbit the sun, since the electron is spread out in a wave. Furthermore, an electron in a stable atomic state does not move in the sense of waving through space.Electrons move through a wire from the negative end to the positive end. The resistor uses the energy of the electrons around the wire and slows down the flow of electrons. A battery is one way to generate electric current.
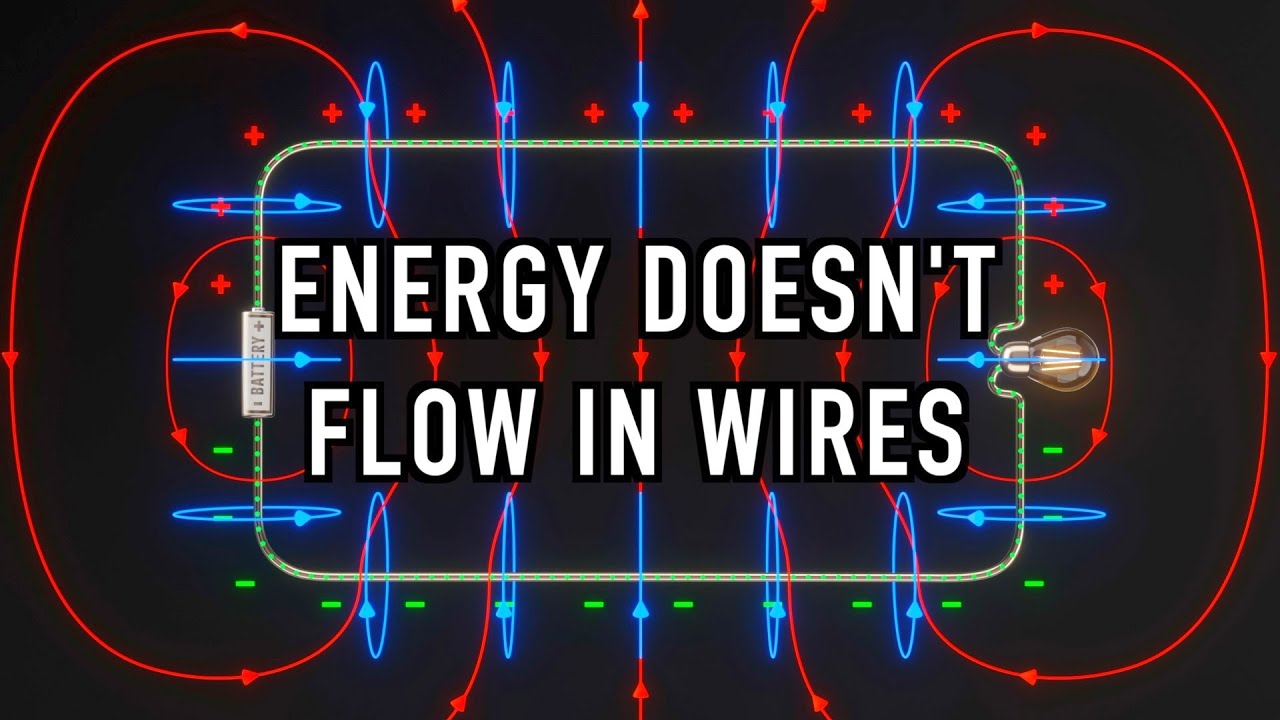
Table of Contents
Are electrons actually moving?
With all of this in mind, an electron in a stable atomic state does not move in the sense of a solid little ball zipping around in circles like how the planets orbit the sun, since the electron is spread out in a wave. Furthermore, an electron in a stable atomic state does not move in the sense of waving through space.
Do electrons move How do they move?
Electrons move through a wire from the negative end to the positive end. The resistor uses the energy of the electrons around the wire and slows down the flow of electrons. A battery is one way to generate electric current.
The Big Misconception About Electricity
Images related to the topicThe Big Misconception About Electricity

What actually causes the electrons to move?
The “electrical pressure” due to the difference in voltage between the positive and negative terminals of a battery causes the charge (electrons) to move from the positive terminal to the negative terminal.
Do electrons move around freely?
While the normal motion of “free” electrons in a conductor is random, with no particular direction or speed, electrons can be influenced to move in a coordinated fashion through a conductive material. This uniform motion of electrons is what we call electricity or electric current.
Do electrons move at the speed of light?
A calculation shows that the electron is traveling at about 2,200 kilometers per second. That’s less than 1% of the speed of light, but it’s fast enough to get it around the Earth in just over 18 seconds. Read up on what happens when nothing can go faster than the speed of light.
Can electrons be at rest?
Could electrons ever exist stably at rest? A: There aren’t any quantum states of electrons or any other little object that are completely at rest.
Do electrons actually move around the nucleus?
The answer is: although it is convenient to think of the electron moving around the nucleus along circular paths, the correct description is a quantum mechanical one.
See some more details on the topic Do Electrons Actually Move? here:
The lights turn on very quickly when I flip the switch. Just how …
The electricity that is conducted through copper wires in your home consists of moving electrons. The protons and neutrons of the copper atoms do not move. The …
What’s electron flow?
Electron flow is what we think of as electrical current. We are familiar with two types of electron flow, Direct Current, or DC, and Alternating Current, or AC.
Does an electron in an atom move at all?
With all of this in mind, an electron in a stable atomic state does not move in the sense of a solid little ball zipping around in circles like …
Q & A: Electrons moving in conductors | Department of Physics
Q: We know that electrons are free to move about in a conductor ..they have a drift velocity of 1cm/s , yet when we see …
How does current actually flow?
The direction of an electric current is by convention the direction in which a positive charge would move. Thus, the current in the external circuit is directed away from the positive terminal and toward the negative terminal of the battery. Electrons would actually move through the wires in the opposite direction.
What happens if electrons stopped moving?
Because of the quantum uncertainty principle, electrons cannot be entirely stopped. If an electron were exactly at rest, the uncertainty in its velocity would be zero, implying an infinite uncertainty in its position. Such an extreme state can be approached but never really attainted.
Can electrons be destroyed?
An electron can never be created on its own. Or it takes its charge from other particles, or a positron is created at the same time. Likewise, an electron can’t be destroyed without another equally, but oppositely, charged particle being created. When the electron is isolated, it can never be destroyed.
Does electricity push or pull?
If two things have the same charge they’ll push away from each other, but if they have opposite charge they’ll pull toward each other. Physicists usually talk about this by saying that charged things produce a positive or a negative electric field, and that this electric field pushes or pulls other charged things.
Does Electricity REALLY Flow? (Electrodynamics)
Images related to the topicDoes Electricity REALLY Flow? (Electrodynamics)

Can a wire run out of electrons?
Circuits don’t create, destroy, use up, or lose electrons. They just carry the electrons around in circles. For this reason, circuit electrical systems can’t really run out of electrons. The energy delivered through a circuit is not the result of electrons existing in the circuit.
Are atoms always in motion?
The word kinetic refers to motion and the kinetic molecular theory suggests that atoms and molecules are always in motion. The energy associated with this motion is termed kinetic energy.
Do electrons experience time?
All Electrons and all Protons experience Time Dilation and Lenght Contration, but this is only adecuatelly defined just when done with respect to another Systems of Reference, another reference Observers), this is Relativity.
Is darkness faster than the speed of light?
Darkness Is Faster Than the Speed of Light.
Is anything faster than light?
So, according to de Rham, the only thing capable of traveling faster than the speed of light is, somewhat paradoxically, light itself, though only when not in the vacuum of space. Of note, regardless of the medium, light will never exceed its maximum speed of 186,282 miles per second.
Is light faster than electricity?
Light travels through empty space at 186,000 miles per second. The electricity which flows through the wires in your homes and appliances travels much slower: only about 1/100 th the speed of light.
Are atoms ever at rest?
Even if we ignore every kind of field and particle except electrons, protons and neutrons, we find that atoms are still not empty. Atoms are filled with electrons. It’s true that a large percentage of the atom’s mass is concentrated in its tiny nucleus, but that does not imply that the rest of the atom is empty.
What is the electron made of?
The Atom Builder Guide to Elementary Particles
Atoms are constructed of two types of elementary particles: electrons and quarks. Electrons occupy a space that surrounds an atom’s nucleus. Each electron has an electrical charge of -1. Quarks make up protons and neutrons, which, in turn, make up an atom’s nucleus.
Can a quantum particle be at rest?
Yes of course. From time to time you will also measure the particle with zero momentum. But the definition of being at rest (in the sense of being in an momentum eigenstate with momentum zero) is that you will always measure it with zero momentum and no other outcome is possible.
What Are Electrons REALLY Doing In A Wire? Quantum Physics and High School Myths
Images related to the topicWhat Are Electrons REALLY Doing In A Wire? Quantum Physics and High School Myths
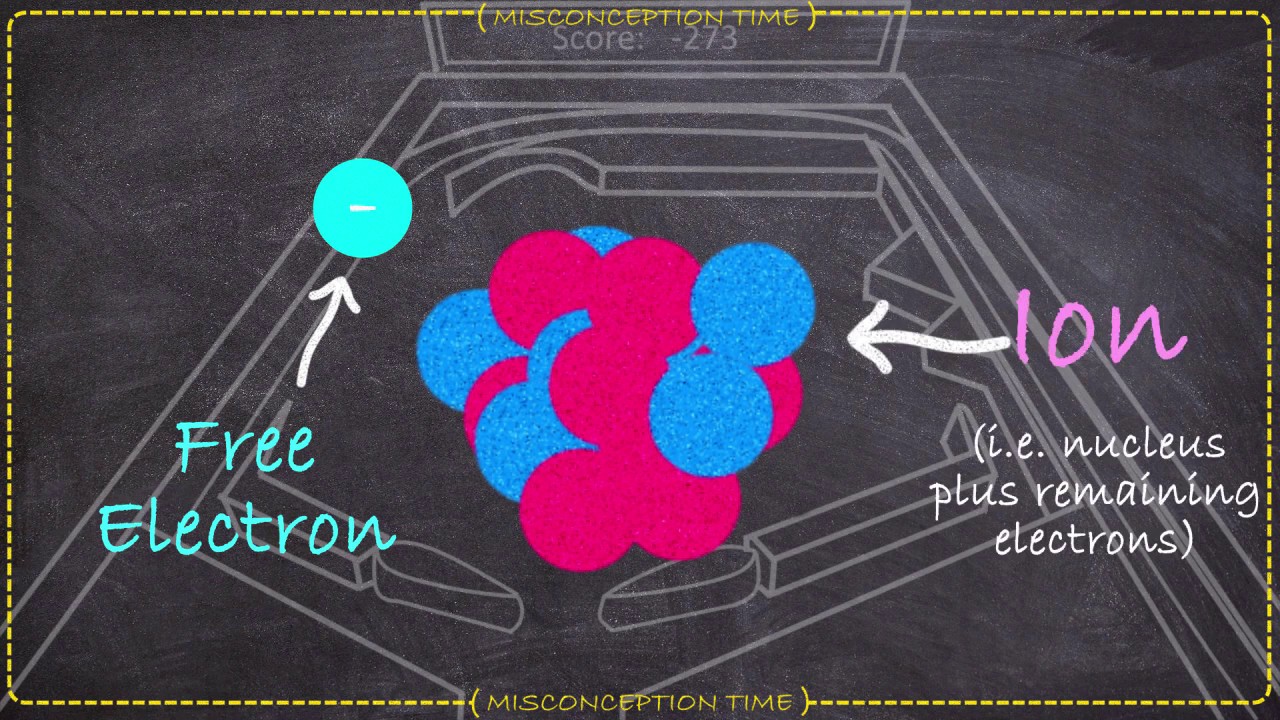
How do electrons stay in orbit?
Like gravity acting on planets, an electromagnetic force attracts the orbiting electron to the nucleus. Classical physicists wondered that the electron didn’t run out of energy. Niels Bohr solved this mystery by introducing quanta, discrete energy states in which electrons may stably persist.
Why electron does not jump into the nucleus?
Quantum mechanics states that among all the possible energy levels an electron can sit in the presence of a nucleus, there is one, which has THE MINIMAL energy. This energy level is called the ground state. So, even if atoms are in a very very called environment, QM prohibits electrons from falling to the nucleus.
Related searches to Do Electrons Actually Move?
- why do electrons flow in the opposite direction of current
- how do electrons actually move
- why do only electrons move
- why do electrons move so easily in conductor
- what happens when electrons stop moving
- why do electrons move and not protons
- do electrons move at the speed of light
- do electrons actually move in a conductor
- what happens when electrons move
- the description for the circuit when the electrons flow from the source and back again
- do electrons move randomly
- do electrons actually move around the nucleus
- how fast do electrons move in a wire
- do electrons actually move in a circuit
- what actually causes the electrons to move?
- how do electrons transfer energy
- how do electrons move
- what makes electrons move
- do electrons move fast
- how electrons move in a wire
Information related to the topic Do Electrons Actually Move?
Here are the search results of the thread Do Electrons Actually Move? from Bing. You can read more if you want.
You have just come across an article on the topic Do Electrons Actually Move?. If you found this article useful, please share it. Thank you very much.
