Table of Contents
What is the equation for coal gasification?
This reaction is a complex process that involves multiple steps. First, coal is heated in the presence of oxygen and steam to produce a mixture of gases. This mixture includes hydrogen, carbon monoxide, carbon dioxide, methane, and other hydrocarbons. The composition of the gas mixture depends on the type of coal used, the temperature, and the pressure of the reaction.
The hydrogen gas produced in this process is a valuable fuel and can be used to generate electricity. Carbon monoxide can also be used as a fuel or as a raw material for the production of other chemicals.
Let’s break down the equation further:
3C: This represents three moles of carbon from the coal. The coal is the primary source of carbon for the gasification process.
O2: This represents one mole of oxygen which acts as an oxidizer, reacting with the carbon to produce carbon monoxide and carbon dioxide.
H2O: This represents one mole of water which acts as a source of hydrogen and also reacts with carbon to produce carbon monoxide and hydrogen.
H2: This represents one mole of hydrogen which is a valuable product of the gasification process. It’s a clean-burning fuel.
3CO: This represents three moles of carbon monoxide which is another valuable product of the gasification process. It can be used as a fuel or raw material.
This equation represents the simplified chemical reaction of coal gasification. It is important to remember that this is a complex process that involves many different chemical reactions. This simplified representation helps to understand the core chemical transformations involved in coal gasification.
What is the gasification method of coal?
You might be wondering how gasification differs from the way most power plants burn coal. Traditional coal-fired power plants use combustion, where coal is completely burned to generate heat, which then produces steam to drive turbines. Gasification, however, takes a different approach. It transforms the coal into a gas with a higher energy density, making it a more efficient fuel source.
Imagine it like this: think of burning wood in a fireplace to keep warm. That’s combustion. Now, imagine you instead turned that wood into charcoal. Charcoal is more concentrated energy and burns hotter than the original wood. Gasification is similar. It’s like taking coal and transforming it into a high-energy “charcoal” that can be used more efficiently for power generation.
What are the reactions related to coal gasification?
Pyrolysis is a fundamental reaction that happens during all gasification processes. It’s like breaking down coal into smaller pieces. This process creates tar, which is a thick, sticky substance.
The tar undergoes two important reactions: hydrocracking and gasification. Hydrocracking is like breaking down the tar even further, using hydrogen to create methane (CH4), hydrogen (H2), and carbon monoxide (CO). Gasification is similar, converting the tar into these same valuable gases.
The char, which is like a solid leftover from the initial breakdown, also undergoes hydrogasification and gasification reactions, producing even more methane (CH4), hydrogen (H2), and carbon monoxide (CO). These gases are important fuels and building blocks for various industrial processes.
It’s important to understand how the different reactions work together to create the valuable gases that are produced by coal gasification. These reactions are essential for turning coal, a solid fuel, into a more versatile and usable energy source.
Think of pyrolysis like baking a cake. The heat from the oven breaks down the ingredients into simpler parts. Tar is like the batter—it’s thick and gooey, and it needs to be broken down further.
Hydrocracking is like adding baking soda to the batter. It helps the batter rise and become lighter. In coal gasification, hydrocracking uses hydrogen to break down the tar into smaller, more useful gas molecules.
Gasification is like the actual baking process, where the heat and the baking soda work together to create a delicious cake. In coal gasification, the heat and the hydrogen work together to transform the tar into valuable gases like methane (CH4), hydrogen (H2), and carbon monoxide (CO).
Finally, the char is like the leftover crumbs after you finish the cake. Even these crumbs can be used, undergoing hydrogasification and gasification to create even more methane (CH4), hydrogen (H2), and carbon monoxide (CO). So, you can see that every part of the coal gasification process is important for creating valuable gases that can be used for various purposes.
What is the composition of coal gasification syngas?
You’re probably wondering what exactly makes up coal gasification syngas, and that’s a great question! The composition of syngas can vary quite a bit depending on the type of coal used and the gasification process itself.
But, typically syngas is made up of:
30 to 60% carbon monoxide (CO)
25 to 30% hydrogen (H2)
0 to 5% methane (CH4)
5 to 15% carbon dioxide (CO2)
And there’s a little bit of water vapor in there, too, along with small amounts of sulfur compounds.
Let’s dive deeper into these components:
Carbon monoxide (CO) is a crucial component of syngas, often making up a significant portion of the mixture. It’s a colorless, odorless gas that’s highly flammable and plays a vital role in chemical synthesis and energy production.
Hydrogen (H2), another key component, is a colorless, odorless, and tasteless gas that’s the lightest element on the periodic table. It’s a clean-burning fuel with a high energy content and is used in various industrial processes.
Methane (CH4), a simple hydrocarbon, is present in smaller amounts in syngas. It’s a potent greenhouse gas, but it can also be a valuable fuel source.
Carbon dioxide (CO2), a greenhouse gas, is also found in syngas. It’s a byproduct of the gasification process and can be captured and stored to mitigate climate change.
The exact proportions of these components depend on factors like the type of coal used, the gasification temperature, and the presence of other gases in the reaction. But understanding these main ingredients gives you a good idea of what makes up syngas and why it’s a valuable resource for energy production and chemical synthesis.
What is the formula for coal gas?
Let’s break down how coal gas is made. When coal is heated in the absence of air, it undergoes a chemical transformation. This process, known as destructive distillation, breaks down the coal into various components, including coal gas. The process is carried out in large ovens called coke ovens, where the coal is heated to high temperatures. This heat causes the coal to decompose, releasing a mixture of gases, including methane, carbon monoxide, and hydrogen.
You might be wondering why coal gas is important. Well, it has a rich history as a fuel source. In the past, it was widely used for lighting, heating, and even powering industrial machinery. Although its use has declined with the advent of natural gas, coal gas remains an important byproduct of the coal industry.
What is coal gasification conversion?
After pretreatment, coal is fed into a gasification reactor. In the reactor, the coal reacts with oxygen and steam, generating a combustible gas. This gas is a valuable energy source.
Air is used to provide the oxygen for producing low-Btu gas. If you want medium-Btu or high-Btu gas, you use pure oxygen. This is because the nitrogen in air dilutes the heating value of the gas.
Think of it like this: Imagine you’re making a fire. If you use a fan to blow air on the fire, it will burn hotter but less efficiently. But if you use pure oxygen, the fire will burn intensely and produce more heat. The same principle applies to coal gasification.
Coal gasification is a process that transforms solid coal into a clean-burning, versatile fuel. The process involves several steps, but the core of it is the gasification reactor. The reactor is where the magic happens – it’s the heart of the conversion process. The type of gas produced depends on the source of oxygen used. Air, with its nitrogen content, produces low-Btu gas. On the other hand, pure oxygen leads to medium-Btu or high-Btu gas. These different types of gas have varying applications, and the choice depends on the specific needs of the user.
See more here: What Is The Gasification Method Of Coal? | Coal Gasification Can Be Represented By The Equation
What is coal gasification?
The syngas produced through coal gasification can be used for various purposes, such as:
Generating electricity – The syngas can be burned in a gas turbine to produce electricity.
Producing fuels – The syngas can be converted into liquid fuels, such as methanol and diesel.
Manufacturing chemicals – The syngas can be used to produce a variety of chemicals, such as ammonia, methanol, and ethylene.
Coal gasification offers several advantages over traditional coal combustion methods, including:
Higher efficiency – Coal gasification is more efficient than burning coal directly, as it can capture more energy from the coal.
Reduced emissions – Coal gasification can significantly reduce greenhouse gas emissions, especially carbon dioxide, compared to traditional coal combustion.
Flexibility – Coal gasification can be used to produce a wide range of fuels and chemicals, making it a flexible technology.
Let’s delve a bit deeper into the heart of heterogeneous gas-solid reactions, the process behind coal gasification. Imagine a scenario where coal (the solid) interacts with gases like oxygen and steam (the gases). These reactions take place on the surface of the coal particles. Think of it like a dance, where the gases “stick” to the coal, causing a transformation. This transformation results in the coal being converted into a syngas, a mixture of carbon monoxide and hydrogen. The heterogeneous gas-solid reactions happening in coal gasification are a complex mix of chemical and physical changes, but it’s this interaction that allows us to unlock the energy potential of coal in a more efficient and cleaner way.
How do you calculate a gasification process?
First, we have the reaction of carbon and oxygen to form carbon dioxide. This reaction is represented by the equation: C + O2 = CO2. The enthalpy change for this reaction is -94.05 kcal/mol, meaning it releases heat.
Next, we have the reaction of hydrogen and oxygen to form water. This reaction is represented by the equation: H2 + 0.5O2 = H2O. The enthalpy change for this reaction is -68.3 kcal/mol, also indicating that it releases heat.
The heat generated from these exothermic combustion reactions is crucial because it powers the endothermic reactions that drive the gasification process. This interplay between heat release and heat absorption is what makes gasification such a unique and effective process.
Now, to understand how we calculate the gasification process, we need to delve into the specific equations and factors involved. The enthalpy changes, also known as heat of reaction, for each reaction are fundamental to calculating the overall energy balance of the gasification process. By considering these enthalpy changes, along with the stoichiometry of the reactions, we can determine the amount of heat required or released during the gasification process.
For example, if we know the amount of carbon and hydrogen in the feedstock, we can calculate the amount of heat released from their combustion. This heat can then be used to calculate the amount of heat available for the endothermic gasification reactions.
The gasification process is complex, involving many different reactions, but understanding these basic principles is essential for making calculations and optimizing the process. We’ll delve into more specific calculations in the next section.
What is the Order of non-catalytic gasification reactivities of Cokes?
We can see that French bituminous coal shows the highest reactivity, followed by Pingshuo gas coal inertinite. Pingshuo gas coal vitrinite, Pingshuo gas coal, Fuxin long-flame coal, Fengfeng meager coal, and Dongshan lean coal exhibit progressively lower reactivities.
Non-catalytic gasification is a process where coke reacts with a gasifying agent, like steam or carbon dioxide, to produce combustible gases, without the use of a catalyst. The reactivity of a coke refers to its rate of reaction with the gasifying agent.
Cokes derived from different coal types have varying reactivities due to their distinct physical and chemical properties. These properties include:
Coal rank: Higher-rank coals (like bituminous coal) tend to have lower volatile matter content and more graphitic structures, resulting in lower reactivities. Conversely, lower-rank coals (like lean coal) have higher volatile matter content and less organized structures, leading to higher reactivities.
Maceral composition: Inertinite, a maceral found in coal, is known for its high carbon content and resistance to gasification. Vitrinite, another maceral, has moderate reactivity. The relative abundance of these macerals in a coal influences the coke’s overall reactivity.
Porosity and surface area: A coke’s porosity and surface area play crucial roles in its gasification behavior. Higher porosity allows for better gas diffusion, facilitating the reaction, while a larger surface area provides more sites for the gasifying agent to interact with.
Ash content: Ash content can affect coke reactivity, with higher ash content often leading to lower reactivity. Ash can block pores and reduce the surface area available for gasification.
Understanding the order of non-catalytic gasification reactivities of different cokes is important in optimizing gasification processes. Choosing the right type of coke for a specific application depends on the desired reaction rate and the properties of the gasifying agent. For instance, if a high reaction rate is desired, a coke with high reactivity, such as French bituminous coal, would be preferred. However, if a more controlled reaction is desired, a coke with lower reactivity, like Dongshan lean coal, might be a better choice.
What is integrated coal gasification combined cycle?
Integrated Coal Gasification Combined Cycle (IGCC) is a power generation technology that uses a cleaner and more efficient process than traditional coal-fired power plants.
The core of IGCC is coal gasification, a process that converts coal into a clean-burning fuel gas. Here’s how it works:
Coal is partially oxidized by reacting it with air, oxygen, steam, or carbon dioxide under controlled conditions.
* This process produces a syngas (synthesis gas) that’s mainly composed of carbon monoxide and hydrogen, along with other gases.
Syngas is then cleaned to remove impurities like sulfur and particulate matter.
* The clean syngas is then burned in a gas turbine, generating electricity.
* The hot exhaust gas from the gas turbine is used to produce steam in a heat recovery steam generator (HRSG).
* The steam drives a steam turbine, which generates additional electricity.
This combined cycle approach allows for higher overall efficiency than traditional coal-fired power plants. The IGCC process also significantly reduces emissions, making it a more environmentally friendly option for coal-based power generation.
Let’s break down the coal gasification process further:
The coal is first crushed and pulverized.
* It’s then fed into a gasifier where it’s reacted with the gasifying agent (air, oxygen, steam, or carbon dioxide).
The gasifier operates at high temperatures and pressures, creating the chemical reactions needed to break down the coal into syngas.
* The syngas is then cooled and cleaned to remove contaminants like sulfur dioxide, hydrogen sulfide, particulates, and heavy hydrocarbons.
The cleaning process involves several stages, including filtration, absorption, and adsorption. This step is crucial to ensure the syngas is clean enough to be burned in the gas turbine.
The syngas produced in the gasifier is a versatile fuel source that can be used for various purposes, such as:
Electricity generation, as in IGCC power plants.
Production of synthetic fuels, like methanol and diesel.
Production of chemicals, like ammonia and hydrogen.
The IGCC technology is still relatively new, but it holds great promise for cleaner and more efficient coal-based power generation. It offers a viable option for countries looking to reduce their carbon emissions while still relying on coal as a major energy source.
See more new information: musicbykatie.com
Coal Gasification: The Equation Behind Energy Production
Coal gasification is a fascinating process that transforms coal, a solid fuel, into a mixture of gases that can be used as a fuel source. It’s all about breaking down the complex structure of coal into simpler molecules, making it easier to use.
Let’s delve into the heart of this process – the equation:
C + H₂O → CO + H₂
This equation, though simple, represents a lot. C stands for carbon, the main component of coal. H₂O is water, which acts as a reactant. CO is carbon monoxide, a flammable gas, and H₂ is hydrogen, another combustible gas.
Now, let’s unpack what’s happening. The equation tells us that when carbon (from coal) reacts with water in a high-temperature environment, it forms carbon monoxide and hydrogen.
But this isn’t the whole story. There’s more to coal gasification than just this simple equation.
Understanding the Process:
Here’s a breakdown of what’s going on:
1. Preparation: Before the actual gasification takes place, coal undergoes some preparation. It’s crushed and dried, ensuring uniform particle size and removing any moisture. This step is crucial for the efficiency of the gasification process.
2. Gasification: This is where the magic happens. The prepared coal is fed into a gasifier, which is essentially a large reactor. Here, the coal is subjected to high temperatures (around 1000°C) and a controlled environment. In the gasifier, the coal reacts with steam (water vapor) or oxygen, leading to the formation of the desired gases.
3. Purification: The gas mixture produced in the gasifier isn’t ready for use just yet. It contains impurities like tar, ash, and sulfur compounds. These are removed through a purification process, making the gas clean and suitable for combustion.
4. End Product: The purified gas, commonly known as syngas (synthetic gas), is a mixture of carbon monoxide, hydrogen, and sometimes methane. Syngas can be used directly as a fuel or further processed to produce various chemicals, including methanol, ammonia, and even gasoline.
Why is Coal Gasification Important?
Now that you’ve got the basics, let’s talk about why coal gasification is a big deal.
1. Cleaner Burning Fuel: Coal gasification is often considered a cleaner alternative to directly burning coal. Syngas, unlike coal, burns more efficiently, resulting in fewer emissions. It reduces the release of harmful pollutants like sulfur dioxide, nitrogen oxides, and particulate matter, contributing to cleaner air.
2. Versatile Fuel Source: Syngas is a flexible fuel that can be used in various applications. It can power gas turbines for electricity generation, be blended with natural gas for industrial use, or serve as a feedstock for the production of chemicals and fuels. This versatility makes coal gasification a valuable technology in a world increasingly reliant on energy and resource management.
3. Resource Utilization: Coal gasification enables us to utilize coal resources more efficiently. It unlocks the potential of coal beyond just burning it, allowing us to create a range of useful products. This is especially significant in regions with abundant coal reserves.
Different Types of Gasifiers
Just like there are different types of cars, there are different types of gasifiers used for coal gasification. Each type has its own unique characteristics and advantages.
Fixed-bed Gasifiers: These gasifiers operate with a stationary bed of coal. The coal is fed from the top, and the gas is collected from the bottom. They are generally simple in design but may be less efficient than other types.
Fluidized-bed Gasifiers: In this type, the coal is suspended in a fluidized bed of gas, allowing for better mixing and heat transfer. This results in more efficient gasification.
Entrained-flow Gasifiers: This type uses high-velocity gas to transport and react with the coal particles, leading to very fast gasification rates.
Environmental Considerations:
While coal gasification offers advantages, it’s important to consider the environmental implications.
1. Carbon Dioxide Emissions: Although coal gasification reduces some pollutants, it still produces carbon dioxide. The CO₂ capture and storage (CCS) technology is a vital aspect of mitigating the environmental impact of coal gasification.
2. Waste Management: Coal gasification generates ash and other byproducts that need to be disposed of responsibly. Effective waste management strategies are essential to minimize environmental harm.
3. Water Usage: The gasification process requires significant amounts of water, which can raise concerns about water availability in certain regions.
FAQs about Coal Gasification
Q: Is coal gasification a new technology?
A: Coal gasification isn’t a new idea. It’s been around for centuries, but the technology has evolved significantly over time. Modern gasifiers are more efficient and environmentally friendly.
Q: Is coal gasification the solution to our energy problems?
A: Coal gasification can be a part of the solution, but it’s not a silver bullet. It’s important to consider the environmental impact and explore other renewable energy sources to achieve a sustainable energy future.
Q: Can coal gasification be used for clean energy?
A: Coal gasification can be a cleaner way to utilize coal resources. However, achieving clean energy requires further advancements in technology, particularly in carbon capture and storage.
Q: What is the future of coal gasification?
A: Coal gasification is likely to play an important role in the energy sector, especially in regions with abundant coal resources. The technology is constantly evolving, with ongoing research and development focused on enhancing efficiency and reducing environmental impact.
Coal Gasification – an overview | ScienceDirect Topics
Gasification of coal is a process in which coal is partially oxidated by air, oxygen, steam or carbon dioxide under controlled conditions to produce a fuel gas. The hot fuel gas is ScienceDirect
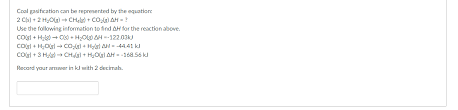
Solved Coal gasification can be represented by the equation
Question: Coal gasification can be represented by the equation: 2 C(s) + 2 H2O(g) → CH4(9) + CO2(g) AH = ? Use the following information to find AH for the reaction above. Chegg
Solved Coal gasification can be represented by the | Chegg.com
Coal gasification can be represented by the equation: 2 C (s) + 2 H2O (g) ? CH4 (g) + CO2 (g) ?H = ? Use the following information to find ?H for the reaction above. C (s) + H2O Chegg
Coal Gasification | SpringerLink
Coal gasification is an important industrial process for converting raw coal into more useful and cleaner carbon feedstocks for use in power generation and as Springer
Gasification of coal – IspatGuru
The major gasification reactions which take place are (i) water gas shift reaction, (ii) Boudouard reaction, (iii) shift conversion, and (iv) methanation. In water gas IspatGuru
Solved Coal gasification can be represented by the equation
Science. Chemistry questions and answers. Coal gasification can be represented by the equation: 2 C (s) + 2 H2O (g) – CH4 (g) + CO2 (g) AH = ? Use the following information Chegg
Coal gasification can be represented by the equation: 2C(s)
The production of syngas from carbon and water and air is known as the coal gasification. The syngas is mixture of some gases, that are carbon monoxide (CO), natural gas: Homework.Study.com
5.1.3. Detailed Gasification Chemistry | netl.doe.gov
Detailed Gasification Chemistry. The chemical reactions of gasification can progress to different extents depending on the gasification conditions (like temperature and National Energy Technology Laboratory
Coal Gasification
Coal Gasification
Coal Gasification Simulation Using Factsage 7.2
C.2 Coal Gasification And Liquefaction (Sl)
Gasification Animation
Numerical Simulations Of Gasification
C2 Coal Gasification And Liquefaction [Sl Ib Chemistry]
C.2 Coal Gasification And Liquefaction (Sl)
Link to this article: coal gasification can be represented by the equation.
See more articles in the same category here: https://musicbykatie.com/wiki-how/
