Table of Contents
Is isobutyl alcohol and sec-butyl alcohol the same?
Let’s explore this further. Imagine you have building blocks, and you can arrange them to create different shapes. In this case, both isobutyl and sec-butyl alcohol are like those building blocks, but the way they’re put together makes a big difference.
Isobutyl alcohol has a branched structure, meaning the carbon chain is not linear. This branching affects the way the molecule interacts with other compounds. In contrast, sec-butyl alcohol has a straight chain with a side branch, making it more linear. This difference in structure can lead to very different reactions, even when using the same chemical reagent.
Think of it like this: if you have a group of LEGO bricks, you can build a tall tower or a wide, flat structure. Even though you use the same bricks, the final product is completely different because of how you arrange them. Similarly, isobutyl alcohol and sec-butyl alcohol have the same atoms, but the way they’re connected leads to different chemical properties.
In summary: Isobutyl and sec-butyl alcohol are isomers—they have the same chemical formula but different structures. This structural difference means they can react with the same reagent to form different products. Just like LEGOs, the arrangement of the building blocks makes a big difference in the final structure and functionality.
What is the difference between isobutyl and tert-butyl structure?
We get the isobutyl group by removing a hydrogen atom from a primary carbon atom in isobutane. It’s considered a primary alkyl group because the carbon atom connected to the rest of the molecule (the one where the hydrogen was removed) is only bonded to one other carbon atom.
The tert-butyl group, on the other hand, forms when we remove a hydrogen atom from the tertiary carbon atom in isobutane. This makes it a tertiary alkyl group because the carbon atom connected to the rest of the molecule is bonded to three other carbon atoms.
Here’s a way to visualize the difference:
Isobutyl can be pictured as a branched chain with three carbons. The carbon at the end of the branch (the primary carbon) is where the hydrogen is removed.
Tert-butyl looks like a “T” shape. The central carbon (the tertiary carbon) is where the hydrogen is removed. It’s attached to three other carbon atoms, forming the “T” shape.
Understanding the difference between isobutyl and tert-butyl is crucial for organic chemistry. It helps you predict how these groups will react in various chemical reactions. For example, tert-butyl groups tend to be more stable than isobutyl groups due to the presence of more electron-donating alkyl groups surrounding the central carbon. This makes them less reactive in certain chemical reactions.
These groups are important in various organic compounds. You’ll encounter them in polymers, pharmaceuticals, and even everyday products like gasoline and plastics.
What is the difference between isobutyl bromide and secondary butyl bromide?
The key difference between these two is the location of the branch in their carbon chains. Isobutyl has its branch on the second carbon, whereas sec-butyl has its branch on the first carbon.
Isobutyl bromide, also known as 2-methyl-1-bromopropane, has a structure where a methyl group (CH3) is attached to the second carbon atom in the propane chain, while a bromine atom is attached to the first carbon atom.
Secondary butyl bromide, also known as 2-bromobutane, has a structure where a bromine atom is attached to the second carbon atom of the butane chain.
Here’s a simplified way to visualize this:
Isobutyl bromide: Imagine a straight chain of three carbons with a bromine attached to the first carbon and a methyl group attached to the second carbon.
Secondary butyl bromide: Imagine a straight chain of four carbons with a bromine attached to the second carbon.
This difference in branching affects their chemical properties, including their reactivity and boiling points.
Here’s a breakdown:
Isobutyl bromide:
Structure: Branched at the second carbon.
Formula: CH3CH(CH3)CH2Br
Reactivity: Reacts through SN2 reactions with primary alkyl halides.
Secondary butyl bromide:
Structure: Branched at the first carbon.
Formula: CH3CH2CHBrCH3
Reactivity: Reacts through SN1 reactions with secondary alkyl halides.
Understanding these subtle structural differences is essential for predicting how these compounds will behave in various chemical reactions.
What is the difference between ISO and SEC?
Iso is used when all the carbon atoms in the chain form a continuous long chain, and one carbon atom is not part of that chain. This additional carbon is attached to the second-to-last carbon atom in the long chain. In simpler terms, imagine a straight chain with a branch extending from the second-to-last carbon.
Sec is used when the functional group present in the carbon chain is bonded to a secondary carbon atom. A secondary carbon atom is one that is directly attached to two other carbon atoms. This means the functional group is not on the end of the chain, but rather on a carbon atom in the middle.
Here’s a visual analogy to solidify the differences:
Imagine a straight road (the main carbon chain). With iso, you have a side road branching off near the end of the main road. With sec, the side road is branching off from the middle of the main road.
Let’s illustrate with some examples:
Isobutane has the structure: CH3-CH(CH3)-CH3. The iso prefix indicates that the fourth carbon atom in the chain is not part of the main chain and is attached to the second carbon atom in the chain.
Sec-butyl alcohol has the structure: CH3-CH2-CH(OH)-CH3. The sec prefix indicates that the hydroxyl group (OH) is attached to the secondary carbon atom, which is the second carbon atom from the end of the chain.
These prefixes provide valuable information about the structure of organic molecules. They help chemists understand how the functional groups and substituents are arranged in the molecule, which is crucial for predicting its reactivity and properties.
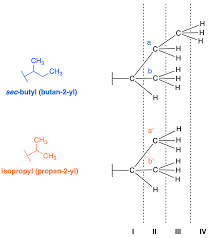
What’s the difference between isobutyl and sec-butyl?
Imagine a chain of four carbon atoms. For isobutyl, the branch (a single carbon) sticks out from the second carbon in the chain. Think of it like a side road branching off the main highway. For sec-butyl, the branch goes off the first carbon in the chain.
Here’s a visual to help:
Isobutyl:
“`
CH3
|
C
/ \
CH3 CH2-CH3
“`
Sec-butyl:
“`
CH3-CH-CH2-CH3
|
CH3
“`
You’ll often see these groups as part of larger molecules, like alcohols or ethers. Understanding their structure is key to figuring out how these bigger molecules will behave.
Let me know if you have more questions about isobutyl and sec-butyl!
What is the difference between secondary alcohol and ISO alcohol?
Isopropyl alcohol is a secondary alcohol with a three-carbon chain. Its molecular mass is 60.10 amu. The IUPAC name for isopropanol is propane-2-ol, represented as (CH3)2CHOH.
Secondary alcohols are organic compounds with a hydroxyl group (OH) attached to a carbon atom that is connected to two other carbon atoms. Isopropyl alcohol is a specific type of secondary alcohol where the hydroxyl group is attached to the middle carbon in a three-carbon chain.
Isopropyl alcohol is commonly called rubbing alcohol and is used as a disinfectant and as a solvent. It is also used as a fuel additive.
Iso alcohol is a general term that refers to any alcohol with an isopropyl group. An isopropyl group is a three-carbon branched chain where the middle carbon atom is attached to two methyl groups (CH3). Isopropyl alcohol is an example of an iso alcohol. Other examples of iso alcohols include isobutyl alcohol and isopentyl alcohol.
Iso alcohols are often used in industrial processes as solvents, anti-freeze agents, and fuel additives. They are also used in the production of plastics, resins, and pharmaceuticals.
What is a suitable test to distinguish between isobutyl alcohol and t-butyl alcohol?
Here’s how the test works:
Tertiary alcohols react very quickly with Lucas reagent, forming a cloudy white solution almost immediately at room temperature. This happens because the tertiary carbocation formed during the reaction is very stable.
Secondary alcohols are a bit slower to react, taking about 3 to 5 minutes to turn cloudy when heated. This is because the secondary carbocation is less stable than a tertiary carbocation.
Primary alcohols like isobutyl alcohol, however, don’t react at all with Lucas reagent under normal conditions. This is because the primary carbocation is the least stable of the three.
t-Butyl alcohol is a tertiary alcohol, so it will react quickly with Lucas reagent and turn cloudy right away. Isobutyl alcohol, being a primary alcohol, won’t react at all. This makes the Lucas test a simple and effective way to distinguish between these two alcohols.
See more here: What Is The Difference Between Isobutyl And Tert-Butyl Structure? | Difference Between Isobutyl And Sec Butyl
What is the difference between isobutyl & sec-butyl?
Isobutyl and sec-butyl both have four carbon atoms, but they differ in how those carbons are arranged. Think of them like Lego blocks – you can put the same blocks together in different ways to build different structures.
Isobutyl has a branched structure, which means it has a side chain. Picture this: a straight chain of three carbon atoms, and then a single carbon atom (a methyl group) branching off the middle carbon.
Sec-butyl has a straight chain structure with a methyl group attached to the second carbon atom from the end. This makes it a bit different from a normal straight-chain butane, where the methyl groups are at the ends of the chain.
To help you visualize this, here’s a simple analogy: Imagine a four-legged table. Isobutyl is like a table where one leg is attached to the middle of another leg. Sec-butyl is like a table where one leg is attached to the side of the table’s frame, but not to the center.
Understanding the difference between isobutyl and sec-butyl can be important in organic chemistry and when you’re working with molecules that have these groups attached.
What is the second form of sec-butyl?
The second form of sec-butyl has the same carbon atom chain arrangement as the first form. The difference is where the rest of the molecule attaches. It connects to the second carbon in the chain. You can see this in its structure: CH3 – CH2 – CH(CH3) -. This is why it’s also called secondary butyl or sec-butyl in common names.
Think of it like this: You have a straight line of four carbon atoms. The first form of sec-butyl has something attached to the first carbon. The second form has something attached to the second carbon.
Here’s a more detailed breakdown:
The first form of sec-butyl (sometimes called isobutyl) is a branched-chain alkyl group with the structure (CH3)2CHCH2.
The second form of sec-butyl (sometimes called secondary butyl) has a straight chain with four carbon atoms and a branch on the second carbon atom. It has the structure CH3CH2CH(CH3).
So, while the carbon chain looks the same, the position of the branch makes a big difference. This difference influences how the molecule interacts with other molecules and affects its chemical properties.
Let me know if you want to explore other forms of butyl groups or have any more questions!
What is the structure of the sec-butyl group?
Let’s break down what that means. In a secondary carbon, the carbon atom is directly bonded to two other carbon atoms. This is in contrast to a primary carbon (bonded to one other carbon) or a tertiary carbon (bonded to three other carbons). In the case of the sec-butyl group, the secondary carbon is the one that’s attached to the rest of the molecule.
The sec-butyl group is an alkyl group, which means it’s a hydrocarbon chain with one carbon atom removed and a single bond to another molecule. It’s commonly found in organic chemistry, and it’s important to understand its structure and how it interacts with other molecules.
For example, let’s consider a molecule of sec-butyl alcohol, which has a sec-butyl group attached to an OH group. The OH group is attached to the secondary carbon of the sec-butyl group. This structure influences the chemical properties of the alcohol.
The sec-butyl group is a great example of how the structure of a molecule can affect its properties and reactivity. Understanding its structure is a key part of understanding organic chemistry.
Why do isobutyl & sec-butyl have different chemical reactivity?
Isobutyl, with its branched structure, is a bit more reactive when it comes to substitution reactions. This is because the methyl group attached to the central carbon is a good target for nucleophiles. Imagine a nucleophile like a hungry shark looking for a tasty bite—that methyl group is like a juicy piece of fish.
Sec-butyl, on the other hand, is less reactive towards substitution reactions because its structure makes it harder for nucleophiles to attack. The secondary carbon with its two attached alkyl groups is sterically hindered, like a big, bulky shark trying to get to that tasty fish. Think of it like this—the bigger the shark, the harder it is to get to that small fish.
Now, let’s dive a little deeper into why the methyl group on isobutyl makes it more reactive. It’s all about stability! When the methyl group is replaced by a nucleophile, the resulting product is more stable than if the nucleophile attacked a secondary carbon like in sec-butyl. This increased stability is because the methyl group is electron-donating, meaning it shares its electrons with the central carbon, which makes it less likely to be attacked by nucleophiles.
In essence, isobutyl is a bit like a small, agile fish, easily caught by a hungry shark (the nucleophile), while sec-butyl is like a big, strong fish, harder to catch. The methyl group on isobutyl makes it a prime target for nucleophilic substitution.
See more new information: musicbykatie.com
Difference Between Isobutyl And Sec-Butyl: A Clear Explanation
These groups are often encountered in the context of alkyl halides, alcohols, and other organic compounds. So, what’s the difference? Let’s break it down.
The Root of the Difference: Carbon Skeletons
The key to understanding the distinction lies in the carbon skeleton. Both isobutyl and sec-butyl are four-carbon alkyl groups, but their arrangement and branching differ.
Isobutyl has a central tertiary carbon (connected to three other carbons), with one methyl group (CH3) directly attached. The other two carbons form a straight chain. It looks like this:
“`
CH3
|
C
/ \
CH3 CH2CH3
“`
Sec-butyl, on the other hand, has a secondary carbon (connected to two other carbons) as its central atom. One of the carbons forms a straight chain with two carbons, while the other is a methyl group attached to the secondary carbon. It looks like this:
“`
CH3CH2
|
CH
/ \
CH3 H
“`
Nomenclature:
Both isobutyl and sec-butyl are named using prefixes to indicate their structure:
Isobutyl gets its name from its “iso” prefix, which signals a branched chain structure with a tertiary carbon.
Sec-butyl gets its name from the “sec” prefix, short for “secondary”, highlighting its secondary carbon.
Impact on Chemical Properties
The subtle structural variations between isobutyl and sec-butyl can lead to differences in their chemical reactivity. This is particularly true when considering reactions that are influenced by steric hindrance.
Steric hindrance refers to the difficulty of a reagent to approach and react with a molecule due to the presence of bulky groups.
Because isobutyl has a more branched structure, it can experience greater steric hindrance compared to sec-butyl. This can affect reaction rates and even product selectivity in some cases.
Examples in Organic Chemistry:
Let’s look at some common examples to illustrate the differences between isobutyl and sec-butyl:
Alcohols: Isobutanol (CH3)2CHCH2OH and sec-butanol CH3CH(OH)CH2CH3 are examples of alcohols containing isobutyl and sec-butyl groups, respectively.
Alkyl halides: Isobutyl bromide (CH3)2CHCH2Br and sec-butyl chloride CH3CH(Cl)CH2CH3 are examples of alkyl halides containing isobutyl and sec-butyl groups, respectively.
FAQs
1. What are some applications of isobutyl and sec-butyl groups in organic chemistry?
Isobutyl and sec-butyl groups are found in various organic compounds with diverse applications.
Isobutyl groups appear in pharmaceuticals, polymers, and fuels. For instance, isobutyl acetate is a common solvent used in paints and coatings.
Sec-butyl groups are present in plasticizers, lubricants, and insecticides. For example, sec-butyl alcohol is a crucial component in the production of certain types of plastics.
2. Are isobutyl and sec-butyl isomers?
Yes, isobutyl and sec-butyl are isomers. Isomers are molecules that have the same molecular formula but different structural arrangements. In this case, both isobutyl and sec-butyl have the same molecular formula, C4H9, but their carbon skeletons differ.
3. How do I distinguish between isobutyl and sec-butyl in a molecule?
To differentiate between isobutyl and sec-butyl groups in a molecule, you can look for the presence of a tertiary carbon in isobutyl and a secondary carbon in sec-butyl. This is the most reliable way to identify them.
4. What is the difference between isobutyl and tert-butyl?
Isobutyl has a tertiary carbon, but it’s not a tert-butyl group.
Tert-butyl is a four-carbon group with a quaternary carbon (connected to four other carbons). It looks like this:
“`
CH3
|
C
/ \
CH3 CH3
“`
5. How can I learn more about isobutyl and sec-butyl?
There are plenty of resources available to delve deeper into the world of isobutyl and sec-butyl. You can find detailed information in organic chemistry textbooks, online tutorials, and scholarly articles. Don’t hesitate to explore and expand your knowledge!
What is the difference between isobutyl and sec butyl? – BYJU’S
Learn the difference between isobutyl and sec-butyl based on their branching position and IUPAC names. See the structures and examples of isobutyl and sec-butyl compounds. BYJU’S
Isobutyl vs. Sec-butyl – What’s the Difference? | This vs. That
Learn how isobutyl and sec-butyl differ in their chemical structure, physical properties, and chemical reactivity. See examples of their applications in various industries and compare thisvsthat.io
Isobutyl vs. Sec-butyl: What’s the Difference?
Learn how isobutyl and sec-butyl differ in structure, reactivity, and applications. See comparison chart, definitions, FAQs, and examples of these four Difference Wiki
Common and systematic naming: iso-, sec-, and tert- prefixes
Learn how to name alkanes with different prefixes based on their structure and arrangement. See examples of isobutyl, sec-butyl, and tert-butyl and how they differ Khan Academy
Don’t Be Futyl, Learn The Butyls – Master Organic
The problem comes from the way we write these compounds: i-PrOH, s-BuOH and t-BuOH, which mean isopropyl alcohol, sec-butyl alcohol and tert-butyl alcohol, all correct names, where i-Pr, s Master Organic Chemistry
Butyl group – Wikipedia
Learn about the four isomers of butyl group, derived from n-butane and isobutane, and their names and structures. Find out the difference between sec-butyl and isobutyl, and how Wikipedia
What is the difference between isobutyl and sec
Hence, the basic difference between isobutyl and sec-butyl is the arrangement of carbon atoms to the parent molecule. Note: Both compounds namely isobutyl and sec-butyl can react with the same Vedantu
Naming Butyls – What does n-, s-, t- Mean? – ThoughtCo
Learn how to name the four forms of butyl group: n-, s-, t-, and isobutyl. See the common and systematic names for each form and their structures. ThoughtCo
Butyl Group – Introduction, Butyl Structure,
The primary distinction between isobutyl and sec-butyl is that the isobutyl group has a branched structure at the second carbon atom of the carbon chain, whereas the sec-butyl group has a branched structure at BYJU’S
Alkane Nomenclature 3 – Sec, Iso, Tert, Neo Naming
Isobutyl Secondary Butyl And Isopropyl Secondary Propyl Are Same Or Different ?
Common Names: Iso, Sec, Tert, Neo, N | Organic Chemistry
006 Alkyl Substituent Names And Structures
Naming Iso, Sec, \U0026 Tert R-Groups
Trick For N Butyl Isobutyl Sec Butyl Tert Butyl | N Butyl Isobutyl | Sec Butyl And Tert Butyl
Trick Using Prefixes (Iso,Sec,Tert,Neo) In Organic Chemistry – Iit Jee \U0026 Neet | Atp Star
Naming Branched Substituents Isopropyl Tert Butyl Isobutyl And More
Link to this article: difference between isobutyl and sec butyl.
See more articles in the same category here: https://musicbykatie.com/wiki-how/
