Table of Contents
Which is the major product of ortho-nitrophenol or para nitrophenol?
Ortho-nitrophenol is favored over para-nitrophenol because of a special interaction called intramolecular hydrogen bonding. Imagine the OH group (hydroxyl group) in ortho-nitrophenol as a tiny hand reaching out to grab the oxygen atom of the nitro group. This grip, or hydrogen bond, creates a strong bond within the molecule itself, making it more stable.
Para-nitrophenol, on the other hand, can’t do this. The nitro group and the OH group are too far apart to form this special bond. This lack of intramolecular hydrogen bonding leaves para-nitrophenol slightly less stable compared to its ortho counterpart. As a result, ortho-nitrophenol is the major product in many reactions.
Think of it like this: If you had two chairs, one with a sturdy backrest and the other without, which one would you choose? The chair with the backrest (like ortho-nitrophenol) is more stable and comfortable, just like the chair without a backrest (like para-nitrophenol) is less stable.
Let’s take a closer look at the nitration of phenol:
When we react phenol with nitric acid, we’re actually adding a nitro group (NO2) onto the benzene ring. The nitro group can attach to different positions on the ring, giving us ortho-nitrophenol, para-nitrophenol, and meta-nitrophenol.
However, ortho-nitrophenol is usually the main product. Here’s why:
The directing effect of the OH group: The OH group on the phenol ring is an ortho-para director. This means it tends to guide the incoming nitro group to the ortho and para positions, but the ortho position is favored.
The stability of the intermediate: The intermediate formed during the ortho nitration is more stable due to intramolecular hydrogen bonding. This makes the ortho product more likely to form.
Kinetic and thermodynamic control: The formation of ortho-nitrophenol is favored both kinetically (faster reaction) and thermodynamically (more stable product).
In conclusion, the combination of the OH group’s directing effect, the stability of the ortho intermediate, and the kinetic and thermodynamic factors contribute to ortho-nitrophenol being the major product of nitration of phenol.
What is the major product when phenol is reacted with dilute hno3?
The reaction proceeds through a series of steps, starting with the protonation of the nitric acid. This protonated nitric acid then acts as an electrophile and attacks the aromatic ring of phenol. The attack can occur at either the ortho or para positions, but the ortho position is favored due to the electron-donating effect of the hydroxyl group.
However, it’s important to note that the reaction doesn’t solely produce ortho-nitrophenol. A small amount of para-nitrophenol is also formed. This is because the para position is also activated by the hydroxyl group, albeit to a lesser extent than the ortho position.
The relative amounts of ortho-nitrophenol and para-nitrophenol formed depend on the reaction conditions. For example, increasing the temperature can favor the formation of para-nitrophenol, while using a stronger acid can increase the rate of the reaction and potentially lead to the formation of other products.
Here’s a breakdown of the factors influencing the formation of ortho-nitrophenol as the major product:
Electron-donating effect of the hydroxyl group: The hydroxyl group in phenol makes the aromatic ring more electron-rich, making it more susceptible to electrophilic attack.
Steric hindrance: The ortho position is more sterically hindered than the para position. This means that the attacking electrophile has a harder time reaching the ortho position, which explains why ortho-nitrophenol is the major product.
Reaction conditions: Factors like temperature, concentration of reactants, and the presence of catalysts can all influence the ratio of ortho-nitrophenol to para-nitrophenol formed.
Understanding these factors is crucial for controlling the outcome of the reaction and obtaining the desired product.
What are the major products in the nitration of phenol?
When you react phenol with dilute nitric acid, you get a mixture of ortho-nitrophenol and para-nitrophenol. Ortho-nitrophenol is the major product, making up about 30% to 40% of the final mixture.
Why is ortho-nitrophenol the major product? It all comes down to the directing effects of the hydroxyl group (-OH) on the phenol ring. The hydroxyl group is an ortho-para director, meaning it directs incoming groups to the ortho and para positions on the ring.
Let’s delve a little deeper.
The hydroxyl group is electron-donating, which means it makes the ring more electron-rich. This electron-richness makes the ortho and para positions more susceptible to electrophilic attack.
Think of it like this: the hydroxyl group is like a magnet, attracting the positively charged nitric acid to the ortho and para positions.
Now, you might be wondering why ortho-nitrophenol is the major product, even though both ortho and para positions are activated. This is because the ortho position is slightly more reactive than the para position. This is due to the proximity of the hydroxyl group to the ortho position.
The close proximity allows for a stronger interaction between the hydroxyl group and the incoming nitric acid, leading to a higher rate of reaction at the ortho position.
So, while both ortho-nitrophenol and para-nitrophenol are formed, the higher reactivity of the ortho position leads to its formation as the major product.
What is the major product of phenol?
Phenol reacts with sodium hydroxide to form sodium phenoxide. The phenoxide ion is more reactive than phenol itself, making it a prime target for electrophilic substitution. When carbon dioxide, a weak electrophile, reacts with phenoxide ion, the result is salicylic acid. This is a significant reaction because salicylic acid is a precursor to aspirin, a common pain reliever.
Salicylic acid is a carboxylic acid with a hydroxyl group attached to the aromatic ring. It’s a versatile compound with various applications, including:
Medicine: As mentioned, salicylic acid is the precursor to aspirin, a widely used pain reliever and anti-inflammatory drug. It is also used in topical medications for acne and other skin conditions.
Agriculture:Salicylic acid is a plant growth regulator, promoting resistance to various stresses like pathogens and drought.
Cosmetics: It’s often found in skincare products due to its exfoliating and anti-inflammatory properties.
The reaction of phenol with carbon dioxide in the presence of sodium hydroxide is a crucial step in the synthesis of salicylic acid. This process is a classic example of electrophilic aromatic substitution, where the phenoxide ion acts as a nucleophile and reacts with the electrophilic carbon dioxide. This reaction highlights the versatility of phenol and its ability to be transformed into valuable compounds.
What is the major product of nitration of chlorobenzene?
Nitration of chlorobenzene is a fascinating chemical reaction that leads to the formation of two primary products: 1-chloro-2-nitrobenzene and 1-chloro-4-nitrobenzene. You might be wondering why these two specific products are formed. Let’s break it down:
The nitration process involves adding a nitro group (-NO2) to a molecule. When we nitrate chlorobenzene, the nitro group can attach to different positions on the benzene ring. However, the chlorine atom attached to the benzene ring influences where the nitro group will go.
Chlorine is considered an electron-withdrawing group because it pulls electrons away from the benzene ring. This effect makes the ortho and para positions on the benzene ring more reactive. As a result, the nitro group is more likely to attach to these positions.
1-chloro-2-nitrobenzene is formed when the nitro group attaches to the position ortho to the chlorine atom. In contrast, 1-chloro-4-nitrobenzene is formed when the nitro group attaches to the position para to the chlorine atom.
Why is the para product favored?
You might notice that the para product is often favored in this reaction. This is because the para position offers a bit more space for the bulky nitro group to attach. Think of it like this: the ortho position is a little cramped, while the para position has more room to breathe.
What about the meta position?
You might be wondering why we don’t see much of the meta product, 1-chloro-3-nitrobenzene. The reason is that the meta position is less reactive due to the electron-withdrawing nature of chlorine.
The Bottom Line
The nitration of chlorobenzene is a great example of how the substituents on a benzene ring can influence the reactivity and regioselectivity of electrophilic aromatic substitution reactions. The ortho and para positions are the most reactive, and the para product is often favored due to steric factors.
What is the mechanism of nitration of phenol?
Nitration is a classic electrophilic aromatic substitution reaction. The key to understanding it is that the electrophile attacks the phenol ring, forming nitrophenol. This happens when we treat phenol with dilute nitric acid.
Here’s how it works:
1. Formation of the electrophile: Nitric acid (HNO3) reacts with sulfuric acid (H2SO4) to generate the nitronium ion (NO2+), which is our electrophile.
2. Attack on the phenol ring: The nitronium ion, being electron-deficient, is attracted to the electron-rich phenol ring. The nitronium ion attacks the ortho and para positions of the phenol ring. This is because the hydroxy group (OH) on the phenol ring is an electron-donating group, making these positions more susceptible to electrophilic attack.
3. Formation of the intermediate: The nitronium ion forms a sigma complex with the phenol ring. This intermediate is stabilized by resonance and is highly reactive.
4. Loss of a proton: The sigma complex loses a proton from the carbon atom where the nitronium ion attacked, resulting in the formation of nitrophenol.
Why do we get ortho and para isomers?
The hydroxy group on the phenol ring is an ortho/para director, meaning it directs the incoming electrophile to the ortho and para positions. This is because the hydroxy group donates electrons to the ring through resonance, increasing the electron density at these positions. This makes these positions more attractive to the electrophile.
Key takeaways:
Nitration of phenol is an electrophilic aromatic substitution reaction.
The electrophile (NO2+) attacks the phenol ring at the ortho and para positions.
* The reaction is catalyzed by sulfuric acid.
* The ortho/para directing effect of the hydroxy group is crucial to understanding the product formation.
Remember, this is just the basic mechanism. There are other factors that can influence the reaction, like temperature and the concentration of reactants. But understanding the fundamental steps is a great starting point for exploring the fascinating world of organic chemistry!
What is the major product of sulphonation of phenol?
Let’s dive into why this happens! It’s all about the balance between kinetics and thermodynamics. At lower temperatures, the reaction is kinetically controlled, meaning the product formed fastest is the major one. The ortho position is more accessible for the electrophilic attack of the sulfur trioxide (SO3) from fuming sulfuric acid. This leads to a faster formation of ortho-phenol sulfonic acid.
As we increase the temperature, the reaction becomes thermodynamically controlled. This means the product with the highest stability is the major one. The para position is more stable due to the electron-donating effect of the hydroxyl group, which helps to stabilize the positive charge that develops during the electrophilic attack.
This change in the major product at different temperatures is a common phenomenon in organic chemistry. It showcases the influence of reaction conditions on the outcome of a reaction.
See more here: Which Is The Major Product Of Ortho-Nitrophenol Or Para Nitrophenol? | Nitration Of Phenol Major Product
See more new information: musicbykatie.com
Nitration Of Phenol Major Product | What Is The Major Product Of Nitro Phenol?
why is o nitrophenol a major product in nitration of
Q. Phenol reacts with sodium hydroxide to give sodium phenoxide. Phenoxide ion undergoes electrophilic substitution with carbon dioxide ( a weak electrophile) because phenoxide ion is more reactive than phenol. BYJU’S
7.4.5 Nitration & Bromination of Phenol – Save My Exams
Nitration is an example of an electrophilic substitution reaction; The nitration of benzene requires a mixture of concentrated nitric acid (HNO 3) and sulfuric acid (H 2 SO 4) savemyexams.com
The mechanisms of nitration of phenol – ScienceDirect
Nitrosation is an electrophilic aromatic substitution involving the nitrosonium ion and is mainly para selective (refs. 4, 5), and the oxidation is due to the nitrogen ScienceDirect
Nitration of Phenol (A-Level Chemistry) – YouTube
Outlining the nitration of phenol reaction. Differences between the nitration of benzene and the nitration of phenol are shown and explained.Recap: 00:25Nitr… YouTube
Electrophilic Substitution Reactions of Phenols
Nitration of Phenols. Phenols upon treatment with dilute nitric acid undergo nitration at low temperature (298 K) to give a mixture of ortho and para nitrophenols. The mixture formed is further separated into ortho BYJU’S
Why is the para product major in the nitrosation of
When phenol reacts with nitrous acid ($\ce{NaNO2 + \mathrm{conc}\ H2SO4}$), 4-nitrosophenol and not 2-nitrosophenol is formed. I cannot understand why the para -isomer should be preferred Chemistry Stack Exchange
Nitration of Phenols (video) | Khan Academy
About. In this video, we will learn how to prepare p-nitrophenol, a very important intermediate in the synthesis of the drug, paracetamol. Khan Academy
22.6: Electrophilic Substitution of Phenols – Chemistry LibreTexts
Direct nitration of phenol (hydroxybenzene) by dilute nitric acid gives modest yields of nitrated phenols and considerable oxidative decomposition to tarry Chemistry LibreTexts
The mechanisms of nitration of phenol – ScienceDirect
Nitration of phenol is an old reaction that was described for the first time in 1875. Nitrophenols are of great interest for the industry because they can be used as ScienceDirect
Nitration of phenols: A two-phase system | Journal of Chemical
Silica Chloride/NaNO 2 as a Novel Heterogeneous System for the Nitration of Phenols Under Mild Conditions. Phosphorus, Sulfur, and Silicon and the Related ACS Publications
Nitration Of Phenols| Electrophilic Aromatic Substitution | Organic Chemistry | Khan Academy
What Is The Major Product In The Nitration Of Phenol
Nitration Of Phenol (A-Level Chemistry)
Bromination Of Phenols | Electrophilic Aromatic Substitution | Organic Chemistry | Khan Academy
Nitration Of Phenol By Hno3 | Phenol Chemical Rxn. | 12Th Organic | Neet Jee Aiims
Nitration Of Methylbenzoate And Nitration Of Bromobenzene
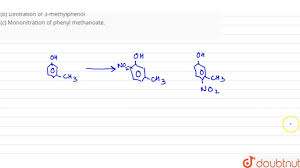
What Is The Major Product(S) Formed By The Mononitration Of 3-Methylphenol? (A) (B) (C) (D) Both…
Nitration Of Phenol | Reactions Of Phenol | Nitration Reaction
Link to this article: nitration of phenol major product.
See more articles in the same category here: https://musicbykatie.com/wiki-how/
