Table of Contents
How do gas particles exert pressure?
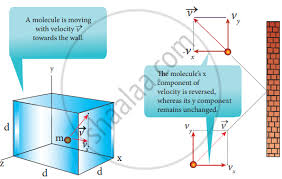
Imagine a tiny gas particle, like a tiny ball, bouncing around inside a box. Each time it hits the wall of the box, it exerts a small force. Since there are millions of these particles all bouncing around, the total force on the walls adds up. Pressure is just a way to measure how much force is being exerted on each unit of area of the wall.
Here’s a simple way to think about it:
More particles: The more gas particles there are in the container, the more collisions there will be, and the higher the pressure.
Faster particles: If the gas particles are moving faster, they will hit the walls harder, leading to more force and higher pressure.
Smaller container: If you squeeze the gas into a smaller container, the particles will collide with the walls more often, increasing the pressure.
That’s how gas particles exert pressure. It’s all about the collisions they make and the force they generate!
How do gas particles create pressure in a container?
The pressure inside the box comes from all these tiny forces added together. Think of it like this: the more balls you have bouncing around, the more often they’ll hit the walls, and the greater the pressure will be. This is why pressure increases when you add more gas to a container.
Also, the faster the balls are moving, the harder they’ll hit the walls, and the higher the pressure will be. This is why pressure increases when you heat up a gas – the molecules move faster!
The pressure is defined as the sum of the forces of all the molecules striking the wall divided by the area of the wall. This means that the pressure is higher when the force per unit area is higher.
So, to summarize, gas pressure is created by the constant bombardment of gas molecules on the walls of a container. The more molecules there are, and the faster they are moving, the more pressure they will exert.
How the gas particles exert a pressure on the walls of the container?
Think about it this way: the more gas particles you have in the box, the more collisions there will be, and the greater the pressure. Similarly, if the particles are moving faster (meaning they have more energy), they’ll hit the walls harder, leading to higher pressure. This is why pressure increases as you heat up a gas—the particles become more energetic and collide with the walls more forcefully!
You can also think about how pressure changes when you change the size of the container. If you squeeze the box smaller, the particles have less space to move around in, and they’ll collide with the walls more frequently. This means the pressure inside the container will increase.
So, pressure is basically a measure of how often and how forcefully gas particles are hitting the walls of their container. It’s a powerful concept that helps us understand how gases behave in different situations!
What causes the pressure exerted by a gas in a container?
It’s important to remember that gas molecules don’t stick together or push each other away. They just bounce off each other and the walls in perfectly elastic collisions, meaning no energy is lost during the collisions. This is why the pressure of a gas remains constant as long as the temperature and volume stay the same.
Let’s break down this idea further. The more molecules you have bouncing around, the more collisions there will be, and the higher the pressure will be. Think about it like this: If you have more people crammed into a small room, they’re going to bump into each other more often, making it feel more crowded.
Similarly, if the molecules are moving faster (like when you heat up the gas), they’ll collide with the walls more frequently and with more force, increasing the pressure. It’s like having everyone in the room running around instead of just standing still.
So, the pressure of a gas is a direct result of the constant bombardment of its molecules on the container walls. It’s a fascinating example of how the tiny world of molecules can have a big impact on the world around us!
How does a gas exert pressure GCSE?
Think of it like this: Imagine you’re standing in a crowded room at a party. Everyone’s bumping into each other, pushing and shoving. That’s kind of like the gas particles in a container. The more people in the room, the more collisions there are, and the more pressure you feel. It’s the same with gas particles. The more particles there are in the container, the higher the pressure will be.
Here’s a little more detail about what’s happening on a microscopic level:
Momentum: When a gas particle collides with the container wall, its momentum changes. Momentum is a measure of how much mass is in motion. Think of it like the force of a moving object. When the particle bounces off the wall, it transfers some of its momentum to the wall, causing a tiny force.
Force and Pressure: Remember, force is a push or pull. Since there are so many particles constantly colliding with the walls, the total force on the walls becomes significant. This force, spread out over the entire area of the container walls, is what we call pressure.
Key takeaway: The pressure of a gas is directly related to the number of particles in the container, their speed, and the size of the container. The more particles there are, the faster they’re moving, and the smaller the container, the higher the pressure will be.
How does a gas exert pressure on its container Quizlet?
These collisions create a force, and the force per unit area is what we call pressure. The more collisions there are, the higher the pressure. This means that factors like the number of gas particles, their speed, and the size of the container all affect the pressure.
Here’s a breakdown:
More particles: The more gas particles there are in a container, the more collisions they’ll have with the walls, and the higher the pressure. This is why increasing the number of moles of gas increases the pressure.
Faster particles: If the gas particles are moving faster, they’ll hit the walls with more force, resulting in higher pressure. This is why increasing the temperature of a gas increases its pressure.
Smaller container: If the container is smaller, the particles will have less space to move around, leading to more frequent collisions with the walls. This is why decreasing the volume of a gas increases its pressure.
These relationships are captured in the ideal gas law, which states that the pressure, volume, and temperature of a gas are all directly related.
How can gas exert pressure on its container?
Imagine yourself as a single gas particle zooming around inside the container. You’re constantly bouncing off other particles and the walls of the container. With every collision, you’re transferring a bit of energy to the wall. Now, imagine thousands upon thousands of these particles all zipping around and colliding with the walls. The combined force of all those tiny collisions adds up to a significant pressure on the container walls.
The more particles there are inside the container, the more collisions there will be, and the higher the pressure will be. Similarly, the faster the particles are moving, the more forceful each collision will be, leading to higher pressure. This is why increasing the temperature of a gas causes it to exert more pressure – the particles gain more energy and move faster.
So, the pressure a gas exerts on its container is a direct result of the constant bombardment of the container walls by the gas particles. The more particles there are, and the faster they move, the more pressure they’ll exert.
How does pressure increase in a container?
The faster the molecules are moving, the more force they exert on the wall of the container, which increases the pressure. It’s all about the energy of those little gas molecules. Think of them like tiny, energetic balls bouncing around inside the container. The more energy they have, the harder they hit the walls.
Here’s a deeper dive into this concept:
Kinetic Energy: When we talk about the energy of the molecules, we’re talking about kinetic energy. This is the energy of motion. The faster the molecules move, the more kinetic energy they have.
Collisions and Pressure: As these energetic molecules bounce around, they collide with the walls of the container. These collisions create force. The more collisions there are, and the more forceful these collisions are, the greater the pressure inside the container.
Temperature and Pressure: There’s a direct relationship between temperature and pressure. As you increase the temperature of a gas, the molecules move faster, increasing their kinetic energy. This leads to more frequent and forceful collisions with the walls of the container, ultimately raising the pressure.
Volume and Pressure: We also have to consider the volume of the container. If you reduce the volume of the container, you’re essentially squeezing the gas molecules together. This makes the molecules collide with the walls more often, leading to a higher pressure.
Think of it like a balloon: If you blow more air into a balloon, you’re adding more molecules. These molecules move around, collide with the balloon’s inner walls, and increase the pressure inside the balloon. That’s why the balloon expands!
See more here: How Do Gas Particles Create Pressure In A Container? | How Do Gas Particles Exert Pressure On Their Container
Why does a gas exert a pressure on the walls of a container?
Each collision is like a tiny push on the wall. The particles don’t just stop when they hit the wall, they bounce back, like a ball hitting a wall. This bouncing back is what creates pressure. Think of it like this: the more collisions there are, the more often the walls are pushed, and the higher the pressure.
So, what causes these collisions? The answer lies in the kinetic energy of the gas particles. They are constantly moving, and their motion is what causes them to bump into the walls of the container. The faster the particles move, the more often they collide with the walls, resulting in higher pressure.
Here’s a cool fact: the pressure of a gas is directly related to the average kinetic energy of its particles. This is known as the kinetic theory of gases. This means that if you increase the temperature of the gas, the particles will move faster and collide with the walls more frequently, leading to higher pressure.
Let’s look at a real-life example. Imagine you have a tire on your bicycle. As you pump more air into the tire, you are adding more gas particles. This means there are more particles to bump into the walls of the tire, leading to a higher pressure. That’s why a bicycle tire feels firm when it’s properly inflated.
How does a gas affect the pressure of a container?
The more of these tiny particles we have in the box, the more often they’ll hit the walls, right? That means more force and higher pressure. This relationship is known as Amontons’ Law, which essentially says that the pressure of a gas is directly proportional to its temperature.
Now, let’s break it down a bit more. The pressure of a gas depends on a few key factors:
The number of gas particles: More particles mean more collisions with the walls, leading to higher pressure. Think about it like this: if you cram more people into a room, it’s going to feel more crowded, right? It’s the same idea with gas particles.
The volume of the container: A smaller container means the particles have less space to move around. This leads to more frequent collisions and higher pressure. Imagine squeezing the box with the bouncing balls – the balls will hit the walls more often.
The speed of the gas particles: Faster particles mean they hit the walls with more force, resulting in higher pressure. Think about how a fast-moving ball hits a wall harder than a slow one.
So, if we want to increase the pressure of a gas, we can either add more gas particles, decrease the volume of the container, or increase the temperature (which increases the speed of the particles).
Think about a bicycle pump. When you push the plunger down, you’re decreasing the volume of the air inside the pump, which increases the pressure. The air then flows out of the pump and into the tire, inflating it.
Understanding how these factors affect pressure is crucial in various applications, from understanding weather patterns to designing engines. It’s a fundamental concept that helps us unlock the secrets of how the world works!
How do gas molecules exert pressure?
Imagine a bunch of bouncy balls in a box. They’re constantly moving around, bouncing off the walls and each other. That’s exactly what gas molecules do. They’re in constant motion, colliding with the walls of their container. Each collision exerts a tiny force, but when you have millions and millions of these tiny forces happening every second, it adds up to a noticeable pressure. The more gas molecules you have in a container, the more collisions, and the higher the pressure.
It’s like if you put more bouncy balls in the box, they’d bump into the walls more often, and you’d feel a stronger force pushing back on the box. That’s why inflating a balloon makes it feel firm – the air inside is pushing outward on the balloon’s rubber.
The pressure a gas exerts also depends on how fast the molecules are moving. The faster they move, the harder they hit the walls, and the higher the pressure. This is why pressure increases when you heat up a gas. Heating the gas gives the molecules more energy, causing them to move faster and hit the walls with more force.
What happens if particles move faster in a container?
Think of it like this: If you gently toss a ball at a wall, it’ll make a soft thud. But if you throw it hard, it’ll make a much louder bang. The same principle applies to the particles in a gas. The faster they move, the harder they hit the container walls, resulting in higher pressure.
Now, let’s talk about what happens when the container walls are flexible, like a balloon. As the pressure inside the container increases, it pushes outwards on the walls. The balloon will expand until the pressure inside is balanced by the pressure outside, which is usually atmospheric pressure.
This expansion is a direct consequence of the increased kinetic energy of the particles. The faster they move, the more energy they have, and the more they push against the container walls. This outward pressure forces the container to expand until the pressure inside is equal to the pressure outside.
Essentially, the container acts like a balancing scale. The pressure inside pushes outwards, while the pressure outside pushes inwards. The container expands until both forces are equal, achieving a state of equilibrium.
See more new information: musicbykatie.com
How Do Gas Particles Exert Pressure On Their Container?
Ever wondered why a balloon inflates when you blow into it? Or why a tire gets firm when you pump air into it? It all comes down to the tiny, energetic particles that make up gases and how they constantly bash against the walls of their container.
Let me break it down for you.
Think about gas particles as tiny, super-fast little balls bouncing around chaotically inside a container. They’re always in motion, zooming in every direction, colliding with each other and with the container walls. These collisions are the key to understanding pressure.
Understanding Pressure
Pressure is basically the force that the gas particles exert on the container walls per unit area. It’s like a tiny army of gas molecules constantly bombarding the walls.
Think about it this way:
– The more gas molecules you have inside a container, the more collisions you’ll have. That means more force pushing against the walls, resulting in higher pressure.
– If you increase the temperature of the gas, those particles will move faster. This means more collisions and greater force on the container walls, leading to higher pressure.
The Big Picture
So, to sum it up, gas particles exert pressure on their container because they’re constantly colliding with its walls. The more collisions and the faster the particles, the higher the pressure.
Here’s a quick breakdown of the relationship between pressure, volume, and temperature for gases:
– Higher Temperature means Higher Pressure (if the volume stays the same).
– Higher Volume means Lower Pressure (if the temperature stays the same).
– Higher Pressure means Lower Volume (if the temperature stays the same).
These are fundamental concepts in gas laws that explain how gases behave under different conditions.
FAQs
#1. What does pressure actually feel like?
Imagine blowing air into a balloon. You can feel the pressure building up inside as the balloon gets bigger. That’s because the air particles are colliding with the inside walls of the balloon, creating that feeling of pressure.
#2. What happens if the pressure gets too high?
If the pressure inside a container gets too high, it can burst or explode. That’s why it’s important to be careful with pressurized containers, like gas tanks or fire extinguishers.
#3. How can we measure pressure?
We use pressure gauges to measure the force of the gas particles pushing on a given area. These gauges are often used in cars, tires, and other situations where pressure needs to be monitored.
#4. Does pressure apply only to gases?
Nope, liquids and solids also exert pressure. For instance, water pressure at the bottom of a pool is greater than the pressure at the surface. But in the case of liquids and solids, the particles are much closer together, making the pressure less dependent on collisions.
In Conclusion
Understanding how gas particles exert pressure is crucial to understanding many physical phenomena around us, from the inflation of a balloon to the operation of a car engine. By comprehending the relationship between pressure, volume, and temperature, we can better appreciate how gases behave and utilize them effectively in various applications.
Gas pressure – BBC Bitesize
Gas pressure is the name given to the force exerted by gas particles colliding with the wall of their container. Pressure is force exerted over an area. Gas pressure is the force… BBC
How does gas exerts pressure on its container? | Socratic
If a gas is heated up, its particles move around more quickly. They hit the walls of their container harder and more often. This increases the pressure. Sometimes Socratic
8.1: Gas Pressure – Chemistry LibreTexts
Gases exert pressure, which is force per unit area. The pressure of a gas may be expressed in the SI unit of pascal or kilopascal, as well as in many other units including Chemistry LibreTexts
13.2: Gas Pressure – Chemistry LibreTexts
Earth’s atmosphere exerts pressure because gravity acts on the huge number of gas particles contained in the atmosphere, holding it in place. Pressure is also exerted by a small sample of gas, Chemistry LibreTexts
6.1: Kinetic Molecular Theory: A Model for Gases
The constant random motion of the gas molecules causes them to collide with each other and with the walls of their container. These collisions of gas molecules with their Chemistry LibreTexts
Particles in gases – OCR Gateway Gas pressure and temperature
Gases and liquids exert pressures on objects and the walls of their containers due to collisions. The greater the force and frequency of these collisions, the greater the BBC
The kinetic molecular theory of gases (video) | Khan Academy
The five main postulates of the KMT are as follows: (1) the particles in a gas are in constant, random motion, (2) the combined volume of the particles is negligible, (3) the particles exert no forces on one another, (4) any collisions between the Khan Academy
Particle motion – Gases – OCR Gateway – GCSE Physics
Gases and liquids exert pressure on objects and the walls of their containers due to collisions. The greater the force and frequency of these collisions, the greater the BBC
Kinetic Molecular Theory – Division of Chemical
The pressure of a gas results from collisions between the gas particles and the walls of the container. Each time a gas particle hits the wall, it exerts a force on the wall. An Division of Chemical Education
Pressure In Gases | Matter | Physics | Fuseschool
Pressure Exerted By Gases | Biology
Why A Gas Exerts Pressure On The Walls Of The Container ?? For Class 9 In Hindi
A Gas Exerts Pressure On The Walls Of A Container In Which It Is Kept. Why ?
Why Gas Particles Exert Pressure On The Wall Of Gas Container
Gcse Physics – Holding Gas In A Container… Pressure And Particles
Gas Particles Pressure – Physics Gcse
How Does A Gas Exert Pressure?
Link to this article: how do gas particles exert pressure on their container.
See more articles in the same category here: https://musicbykatie.com/wiki-how/
